The battery constantly operates in discharge-charge mode. To extend its service life, you should maintain its charge at the maximum level. And to do this, it is necessary to check the battery charge level from time to time. This can be done in different ways, but the most reliable way is to measure the density of the electrolyte. Therefore, many drivers are wondering how to check the battery density.
What is the density of the electrolyte in the battery and what does it affect?
An electrolyte is a special substance that consists of purified water and sulfuric acid (approximately in a ratio of 2:1). The density of the electrolyte, in turn, indicates the ratio of these two components. If you measure this parameter on a new and high-quality product using a special device, the result will be 35/65% (sulfuric acid to water, respectively). Due to the low density of the electrolyte, the battery will not be able to be charged from the generator.
Typically, measuring instruments express this indicator in grams per cubic centimeter. Thus, the measurements tell us how concentrated the acid is in one cubic centimeter of liquid. Automakers make the electrolyte density such that the battery can operate in cold weather with sufficient charging speed and specific power. In this case, concentrated sulfuric acid is not added to the batteries.
What is a hydrometer
A device for measuring the concentration of various solutions is called a hydrometer. The operation of the density meter is implemented on the basis of a well-known physical law.
Archimedes' principle states that if a body is immersed in a liquid, a hydrostatic or lifting force will begin to act on it. In modulus it will be equal to the weight of the liquid that the body displaced, but opposite in direction.
For practical use, the hydrometer scale is graduated to the concentration of the dissolved substance. Car enthusiasts will find a useful device for checking the density of electrolyte and antifreeze.
Normal battery density
The density of the electrolyte in the battery can be measured and this parameter can be adjusted only in serviced batteries. They are produced using WET technology. To describe them in more detail, such batteries are a plastic box divided into 6 compartments. Each of them contains plates filled with electrolyte.
To answer the question of what density should be in a battery, it should be noted that there are several types of batteries. For example, lead-acid batteries are used in many passenger cars, as well as trucks and utility vehicles. As mentioned earlier, the plates of such batteries include ordinary sulfuric acid.
The density of the electrolyte in the jars is measured based on the weight of one cubic centimeter of the composition. On the market you can find electrolytic fluid for filling empty batteries from scratch, with a density of 1.28 g/cm3, as well as a correction substance, the density of which is increased (1.33 g/cm3). In this case, the “normal” density of the battery electrolyte is measured only on a well-charged battery and at a temperature not lower than 23-26 degrees Celsius.
The average value of the described parameter is 1.28 g/cm3. However, operating conditions play a big role. For example, if the car is operated in a cold climate, it is necessary to increase the density of the electrolyte. It should be taken into account that this parameter constantly changes during charges and discharges.
Density table
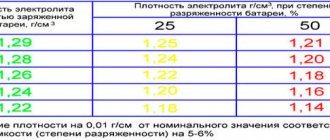
The importance of checking the electrolyte density is difficult to overestimate, since the performance of the car depends on this indicator. Below is a detailed table showing the dependence of the voltage and state of charge of the battery on the concentration of electrolyte in the battery.
| Density at +15 degrees Celsius | No load voltage | Voltage (V) at 100 A load | Charge level | Electrolyte freezing point |
| 1,27 | 12,66 | 10,80 | 100% | -60 |
| 1,26 | 12,6 | 10,65 | 95% | -55 |
| 1,25 | 12,55 | 10,5 | 87,5% | -50 |
| 1,24 | 12,47 | 10,35 | 80% | -46 |
| 1,23 | 12,41 | 10,20 | 75% | -41 |
| 1,22 | 12,35 | 10,05 | 68% | -36 |
| 1,21 | 12,29 | 9,90 | 62,7% | -31 |
| 1,20 | 12,23 | 9,75 | 55% | -27 |
| 1,19 | 12,17 | 9,61 | 50% | -24 |
| 1,18 | 12,11 | 9,45 | 43% | -17 |
| 1,17 | 12,07 | 9,31 | 37,8% | -15 |
| 1,16 | 12 | 9,15 | 32% | -13 |
| 1,15 | 11,95 | 9 | 25% | -12 |
| 1,14 | 11,87 | 8,85 | 19% | -11 |
| 1,13 | 11,82 | 8,67 | 12,54% | -9 |
| 1,12 | 11,77 | 8,55 | 5% | -7 |
| 1,11 | 11,70 | 8,41 | 0 | -6 |
The influence of density on the process of car operation
Fluctuations in electrolyte density have a negative effect on the battery. For example, if this indicator is increased, the water quickly boils away, and concentrated sulfuric acid destroys the plates. This is why car manufacturers recommend regularly adding distilled water to jars.
Low density, in turn, makes starting the power unit more difficult. If the indicator is too low, the substance in the battery may simply freeze. At the same time, such a part cannot be charged, which is why the plates are subject to sulfation. To maintain balance, you need to rely on the practice of increasing and decreasing electrolyte density under different temperature conditions:
- conditions of the Far North – 1.3 g/cm3;
- temperate climate - from 1.24 to 1.28 g/cm3;
- subtropical climate (southern regions of Russia) – from 1.22 to 1.25 g/cm3.
Considering these indicators, it is worth noting that their minimum value cannot be lower than 1.22 g/cm3. If the density is not standardized, the battery will quickly fail and require replacement.
Electrolyte density in winter
A hydrometer is used to measure the density of the battery. This device allows you to find out whether the battery needs corrective electrolyte. Such measurements are especially relevant when operating a car in winter, since in the winter season the density of the liquid in the cans should be from 1.20 to 1.27 g/cm3. It is worth remembering that while in the car, the battery is not fully charged, approximately 80-90%.
When operating a car in conditions where the temperature drops to -50 degrees, the density of the liquid being poured should be in the range from 1.27 to 1.30 g/cm3, with the maximum value being achieved when the battery is fully charged. This is due to two factors:
- If the concentration of H2SO4 (sulfuric acid) is low, the substance in the battery may freeze. Freezing occurs due to large amounts of water.
- In cold weather, all car mechanisms freeze. To maintain their performance, additional energy is required. Therefore, even the most reliable and expensive engine models will not be able to start and operate correctly if the battery is “weak”.
If the winter is warm and the air temperature is about +5 degrees Celsius, the previously described parameter should be equal to 1.22 g/cm3. It is important to constantly monitor this indicator - its drop may lead to the need to replace the battery.
Electrolyte density in summer
In summer, the density of the electrolyte increases as the battery actively loses liquid due to evaporation. For this reason, the indicator should be 0.02 g/cm3 lower than the standard values. It is noteworthy that an increase in temperature under the hood provokes the following processes:
- The liquid from the cans evaporates, and the acid becomes more concentrated.
- The processes of converting electrical energy into chemical energy are accelerated.
A decrease in the volume of electrolyte entails an increase in its density. Distilled water should be added to the battery regularly to control the described parameter. For cars from central regions, such as Moscow, the density of the liquid in the battery should be 1.27-1.28 g/cm3.
Types of device
Since the method of determining density by measuring buoyancy force can be used not only in the case of autonomous power sources, there are several types of hydrometers. They differ both in the value they determine and in their purpose for different types of mixtures.
Since the value of density in the mathematical definition is the ratio of mass to volume, according to the type of measurement performed, devices are divided into hydrometers of constant mass and constant volume.
The scales applied to the floating body allow the device to be used in various areas of practical measurements. For example:
- Salinity meters are devices for determining the concentration of salts in an aqueous solution. They are used, among other things, to find out the degree of soil salinity in different areas.
- Lactometers - measure the fat content of dairy products.
- Alcohol meters - percentage ratio of alcohol and solvent liquid to determine the strength of alcoholic beverages and fermentation products.
- Saccharometers - allow you to find out the degree of concentration of sugars dissolved in water or other liquid.
To increase the accuracy of the operations carried out to measure values, in addition to the drawing with scales printed on the hydrometer, it is equipped with a thermometer. This makes it possible to achieve comparative results on the effect of temperature on a specific liquid and to study the mechanism of its change during warming or cooling.
How to check the battery at home
Operation of the machine must be accompanied by periodic checking of the condition of the battery. As mentioned earlier, an increase in the concentration of sulfuric acid in banks negatively affects the performance of the battery. Car service technicians recommend checking the technical condition of the battery every 15-20 thousand kilometers. Diagnostics can be carried out as follows:
- Inspect the battery case for mechanical damage and leaks.
- Visually assess the electrolytic fluid level. It should rise 1.3 cm above the edge of the jars.
- Measure the electrolyte density using a test instrument.
Sometimes the charge may decrease because the alternator drive belt is loose. Therefore, car owners are recommended to regularly check its tension themselves or at a service center.
Measurements using a hydrometer
As mentioned earlier, measuring the density of the electrolytic substance in the battery is carried out with a hydrometer. Before measuring procedures, it is necessary to assess the level of special fluid in each battery plate and correct it with purified water. After this, the battery needs to be fully charged and the test started, guided by the following algorithm:
- Place the battery on a flat surface.
- Remove the plug on the filler cap.
- Immerse the hydrometer in the electrolytic liquid and draw it into the device using the rubber tip.
- Add enough reagent to move the float.
- Measure the density with a hydrometer, guided by the readings on a special scale.
- Record the result obtained and repeat the procedure on the remaining plates.
- Compare the obtained data with standard values.
It is noteworthy that the values in all banks must be the same. If the measurement shows that the density in individual plates is reduced, it is necessary to check the battery for damage or short circuits between the banks. Low density in all containers may indicate that the battery is severely discharged or worn out. Operating a car with such a battery will be difficult.
Is it possible to take measurements without a hydrometer?
It will not be possible to carry out tests with electrolytic liquid without a special measuring device. At the same time, you can make a hydrometer with your own hands by preparing a float-weight in advance. To create a device yourself, you must perform the following steps:
- Prepare a hollow plastic tube, such as a drinking straw.
- Place the pre-prepared float in purified water, noting the liquid level on it. This mark will correspond to 1 g/ml.
- Place the float in new electrolytic fluid with a precise specific gravity, such as 1.4 g/cm3.
- Make a mark where the electrolyte comes into contact with the float, and then mark the distance between the two available values.
To select the electrolytic fluid, you can use a rubber bulb. As an alternative, a regular glass is used where the float is placed.
Carrying out measurements on a maintenance-free battery
The problem with measuring electrolyte density in maintenance-free batteries is the lack of unscrewable plugs. However, when making batteries, manufacturers use holes that are later sealed with disposable plugs. If necessary, they can be dismantled or drilled, turning a maintenance-free battery into a serviceable one.
It is noteworthy that after servicing the battery, the holes must be securely sealed. This is done using plastic, since it easily melts and sticks to any materials. Therefore, even if it is not possible to completely remove the plugs, they can be drilled and sealed with plastic after servicing.
Video of electrolytic fluid measurements
To understand how the electrolyte density in a battery is measured, just watch the video below:
This video discusses the process of taking measurements using a hydrometer. As mentioned earlier, a similar procedure can be carried out with a homemade measuring tool.
Criteria for choosing a good tool
It all depends on how often you plan to use the battery hydrometer. If checking the battery is rare for you, then a simple hydrometer will suffice - there is no point in spending money on an expensive device. If regular, including preventative, battery checks are a professional necessity for you or a desire to keep the battery in the best possible condition for as long as possible, you should allocate a little more time to the choice.
The first thing you should decide on is the type of device, then its method of measuring density (reference to weight or volume). Then you can pay attention to the manufacturer (relevant only for electrical appliances) and at the very end - to the price. It is not advisable to take a hydrometer that is cheaper than 150-200 rubles (or 500 for a model with electronics).
How to increase battery density
If the fluid level has been regularly adjusted or is not enough to use the battery in winter, it is necessary to increase the density of the electrolyte in the battery. The main sign that it is time to carry out such a procedure is a decrease in the intervals between charging and discharging the battery. To adjust the described indicator, the following devices will be required:
- purchased or homemade hydrometer;
- beaker;
- rubber bulb;
- dishes for diluting new electrolytic liquid;
- purified water;
- corrective acid (or electrolyte).
Next, all methods of increasing the density of electrolytic fluid in a battery will be considered.
How to increase density using charging
This procedure can be carried out using a weak current. To do this you need to do the following:
- Fully charge the battery.
- Wait until the distilled water in the jars begins to evaporate. The overall electrolytic fluid level will decrease.
- Add new electrolyte, the density of which meets the requirements described earlier.
- Take measurements using a hydrometer.
- Repeat the procedure again if you need to adjust the readings.
It is not necessary to throw away the battery if the car stops starting. The part can be made operational again by increasing the density of the electrolyte. The procedure will take a little time, but will save money.
Increase if the density of the electrolytic fluid has dropped below 1.18 g/cm3
If the density drops below 1.18 g/cm3, a correction electrolyte alone is not enough - you need to add acid. The process of adding it is carried out in the same way as in the case of electrolytic liquid, but a small step is taken for this. This is due to the high density of acid, which, when added, can “overshoot” the required mark.
It is noteworthy that if the liquid has acquired a brown tint, it is recommended to replace the battery. Darkening indicates that the active coating on the plates has broken down and dissolved. This means that electromechanical reactions are impossible, as well as increasing the low density of the electrolyte in the battery.
Adding Electrolyte
Another effective way to increase density is to add a correction electrolyte. To carry out this procedure, the following procedure must be followed:
- Taking a small amount of fluid from the battery can.
- Add the same amount of correction fluid. If the density needs to be reduced, distilled water is used instead of the electrolyte.
- Charging the battery with rated current for 30-40 minutes. This is necessary to mix the liquid.
- Disconnecting the battery from charging. After this, it should be left for 1-2 hours to equalize the density and reduce the temperature in all jars.
- Carrying out repeated measurements using a hydrometer.
In order not to encounter problems when carrying out the described procedure, it is necessary to clarify in advance the volume of cans in the battery. You should also completely replace the fluid if the methods listed above do not lead to the desired result.
Electrolyte replacement
If you have no experience in replacing electrolytic fluid, you can use the following instructions. You must first prepare a funnel, a hydrometer, rags, a container for draining the used composition and a rubber bulb. You should also take care of safety precautions by preparing rubber gloves.
To fill in a new correction electrolyte, you need to perform several steps to remove the old composition:
- Remove the battery and place it on a stable surface.
- Unscrew the protective caps and pour the electrolyte from each jar into the prepared container. To do this you need to use a rubber bulb.
- Pour purified water into the jars - this will clear them of any deposits that have formed.
- Shake the battery a little and then drain the water.
- Fill the containers with baking soda and leave them for several hours.
- After removing the soda, pour table salt solution into the jars and wait another hour.
Now that all the above procedures have been completed, it is necessary to prepare a new electrolytic fluid. The procedure for replacing it is as follows:
- Checking the density of a new substance with a hydrometer.
- Slowly pour liquid into jars.
- Removing excess (if any) with a rubber bulb
After replacing the electrolyte, you need to leave the battery alone for 4 days. After this, it can be charged and connected to the car to check its functionality.
Checking the electrolyte level
Before checking the battery density without a hydrometer, you need to set its level. If the battery itself is made of translucent plastic, then checking the electrolyte level is not difficult. If the battery is made of opaque dark plastic, then to check the electrolyte level you will need a special glass tube with a diameter of about 5 millimeters. Such a tube is lowered into the jar until it stops, after which its upper hole is closed with a finger. The tube is carefully removed from the battery. Electrolyte will remain in it, which is poured into the flask and the level is checked. It is believed that the amount of liquid in the flask will be 10-15 millimeters. If the level is higher or lower, it is necessary to level it, and then measure the density of the electrolyte.