What density should the battery have?
To check, a special device called a hydrometer is used. The reference values are obtained at an air temperature of +25°C.
Since ideal conditions are rare, a special table is used in which the density characteristics are adjusted to take into account weather conditions.
Checks should be carried out regularly, at least once every 2 months. Naturally, this is done only on serviced batteries.
Such devices have special twist-out caps that, when removed, allow access to the contents of each jar.
Unscrewed plug from the battery.
The electrolyte density should have an optimal density, but the climate zone must also be taken into account, since the ambient temperature makes its own adjustments to the indicators.
So, in a temperate climate, the electrolyte should have a density of 1.25 to 1.27 g/cu. cm.
The permissible deviation from the specified value is no more than 0.01 g/cubic meter. cm.
In the Arctic zone, where frosts of about -30°C are common in winter, the density should be kept at 0.01 g/cu. cm above, but in the subtropics, on the contrary, by 0.01 g/cubic. see below.
The optimal density in winter and summer is slightly different, as shown in the illustration below:
An interesting feature is that with a lower density in a battery, it lasts longer, but at the same time its capacity decreases.
It should also be taken into account that the above values refer to a 100% charged battery.
In life, the battery has a charge of about 80-90%, respectively, and the density of the electrolyte will be slightly lower.
In practice, in winter, the density of the electrolyte is made a little higher than necessary for a full charge. This is done so that when the temperature drops, which we constantly experience, the battery can still start the engine stably.
In summer it is better not to do this, since the higher the density, the greater the risk of the liquid boiling in the battery.
Attention! Increased electrolyte density leads to a decrease in battery life. However, low voltage reduces voltage and capacity and impairs motor starting. It is best to adhere to the optimal recommended values. During frosts, the density can be increased slightly, but very little.
Battery electrolyte density table
When compiling a table of electrolyte density by climatic zones, we are guided by the average monthly temperatures in January.
For zones with temperatures above -15°C, the density does not change in winter and summer, using the same acid concentration all year round.
It is enough to ensure that the value does not deviate from the required one. But in the Arctic regions, the density requires seasonal changes, otherwise in winter the battery simply will not be able to start the engine.
| Recommended battery electrolyte density | |||||
| Climatic regions | Season | Electrolyte density, normalized to 25°C, g/cm3 | |||
| Filled into the battery | Charged battery | When charging the battery at | |||
| 25% | 50% | ||||
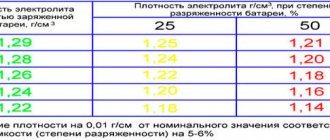
| With a sharply continental climate and winter temperatures below -40°C | Winter | 1.28 | 1.30 | 1.26 | 1.22 |
| Northern with a winter temperature of -40°C | All year round | 1.26 | 1.28 | 1.24 | 1.20 |
| Central with temperatures down to -30°C in winter | 1.25 | 1.27 | 1.23 | 1.19 | |
| Southern | 1.23 | 1.25 | 1.21 | 1.17 | |
Electrolyte density in summer and winter in different climatic regions.
Checking the density of the working fluid
To measure the density of liquids, there are special instruments - hydrometers or density meters. There is one for car batteries as well. It is made in the form of a large syringe, inside of which there is a float with a specially graduated scale.
The autohydrometer float is equipped with a special “syringe” for working in narrow-necked sections of batteries.
In order to measure the density in the battery, plugs are rolled off from all its sections. Next, the hydrometer bulb is compressed, and its needle is immersed in the section. After releasing the bulb, draw electrolyte into the syringe. In this case, the float of the device floats up. The density of the liquid is read from the scale according to the level to which the float floats.
The float rose to level 1.200. Electrolyte density is 1.2 g/cm. cube .
After the measurement, the bulb is squeezed again, and after draining the electrolyte back into the battery, the hydrometer is washed with running water and dried. We should not forget that each section is a separate, independent part of the battery, so the density must be measured in each.
Electrolyte density in the battery in winter
In winter, the density should be 1.27 g / cubic meter. cm. If the January temperature in an area drops to -35 degrees, then the density rises to 1.28 g/cubic meter. cm.
Otherwise, in winter the capacity will decrease and it will become difficult to start the car.
Attention! When the density value drops to 1.09 g/cub. cm results in the fact that the liquid in the battery cans freezes when the thermometer drops to – 7°C. This leads to ruptures and deformations of the hull, destruction of plate packages due to ice expansion.
When the density decreases in winter, this does not mean that you need to increase the concentration of the solution in the jars. You need to recharge the battery with a charger.
The best chargers for car batteries, according to user reviews
Most personal cars are used primarily for commuting to work and shopping centers.
Typically, short distances are covered; when the engine is started, the battery is discharged, and the short travel distance does not allow it to charge normally, especially since the electrolyte is cold due to long periods of downtime outside.
Normal charging occurs only if the electrolyte is warm. Thus, if you do not recharge the battery regularly, its charge level will gradually decrease along with a drop in density.
Therefore, recharging using a charger must also be done in a heated room.
It must be non-residential and have good ventilation. This is important because during the charging process the electrolyte releases gas that is harmful to human health.
Attention! We strongly do not recommend adjusting the electrolyte density yourself! This requires experience. In addition, working with hydrochloric acid is dangerous; this requires compliance with safety precautions. Therefore, you can only add distilled water to the jars yourself if its level drops too low.
It is located at 1.5 cm above the top edge of the plates for cars and 3 cm for trucks.
A newly purchased, new battery should have an electrolyte density of 0.15-0.16 g/m3. cm.
Attention! You cannot use heavily discharged batteries in cold weather; this can lead to freezing of the electrolyte inside, which will at least destroy the plates. But the partitions between the banks or the outer casing may also become deformed.
The table below indicates at what densities and temperatures the electrolyte freezes.
| Electrolyte density, g/cub. cm | Freezing temperature, °C |
| 1.10 | -8 |
| 1.11 | -9 |
| 1.12 | -10 |
| 1.13 | -12 |
| 1.14 | -14 |
| 1.15 | -16 |
| 1.16 | -18 |
| 1.17 | -20 |
| 1.18 | -22 |
| 1.19 | -25 |
| 1.20 | -28 |
| 1.21 | -34 |
| 1.22 | -40 |
| 1.23 | -45 |
| 1.24 | -50 |
| 1.25 | -54 |
| 1.28 | -74 |
Based on the table, it is easy to understand that the battery, even 100% charged, will still freeze if the temperature drops to -70°C.
If it is discharged to 40% of the nominal value, the freezing point will rise to -25°C. Discharging to 10% of the capacity will result in ice appearing inside the cans at -10°C, which is quite a bit for our country.
If there is no hydrometer, then the battery vacuum level is checked using a load fork. In this case, the voltage indicator in different banks should not differ by more than 0.2 V.
| The load fork indicator shows V | Battery discharge level, % |
| 1,8–1,7 | 0 |
| 1,7–1,6 | 25 |
| 1,6–1,5 | 50 |
| 1,5–1,4 | 75 |
| 1,4–1,3 | 100 |
Attention! It’s time to recharge the battery if it is half discharged in winter, and 75% discharged in summer.
Why is a full battery charge necessary?
- The more often the lead plates in the battery become rarefied, the faster the sulfation process occurs.
- The quality of the battery is directly proportional to the density of the electrolyte.
Notes:
- Battery maintenance should be carried out every season. Especially during the autumn-winter period.
- The battery becomes unusable for various reasons. Often with long engine starts using the starter. In winter, the starter consumes increased current and can lead to warping of the plates.
- The charge of the battery is directly proportional to the density of the electrolyte and the degree of charge.
Density of electrolyte in the battery in summer
If in winter the main problem of the battery is a decrease in capacity, then in the summer it is evaporation of the electrolyte.
More precisely, the water evaporates, gradually increasing the concentration of the acid solution. If you do not monitor the level, the liquid will expose the top of the lead plates.
It is impossible to bring them to this state, since they begin to actively deteriorate in the air.
To prevent the acid concentration from increasing too much in hot weather (which is harmful for the plates), the density is often reduced by 0.02 g/cu. see what is considered optimal. Usually in warm weather this does not impair engine starting, but it does extend battery life.
The boiling of water from the electrolyte is facilitated by the fact that it is very hot in the engine compartment in the summer.
The sun heats up the hood lid, and the running engine also gets very hot.
At high temperatures, current output increases and the battery becomes able to easily crank the starter even if the electrolyte density is only 1.22 g/cc. cm (this level is considered the minimum acceptable for a humid, warm climate).
As water evaporates, the liquid level in the jars decreases and the density increases. As a result, the destructive processes of platinum are accelerated.
Therefore, in the summer you need to check the electrolyte level at least once a month, and preferably twice, and periodically add distilled water to the jars.
Otherwise, overcharging will occur due to the high density of the electrolyte, and the plates will undergo accelerated sulfation.
Attention! The increased density of the electrolyte in summer leads to a significant reduction in the service life of the battery.
In summer, charging with a charger is rarely required, but is also used. However, if you have taken out the charger, then at the same time check the condition of the battery - measure the electrolyte level in each jar, add distillate if necessary.
Although mostly water boils away, the acid also evaporates. Therefore, constantly adding distilled water to the solution will eventually cause the concentration to drop.
This leads to the inability of the battery to hold a charge and, accordingly, to operate it. To restore functionality, the density of the electrolyte will need to be increased. To do this you need to check its level.
When and what to top up the battery with?
The need to add working fluid to the battery occurs infrequently, but it is sometimes necessary. What, how much and in what cases should I add? There are two such cases: low electrolyte level and abnormal acidity of the working fluid.
We recommend: How to check battery density at home
Low level in sections
This situation occurs often because during battery operation the water evaporates or, as they say, boils away. In this case, the level of solution in the sections decreases, and the edges of the plates turn out to be dry. This can be determined visually by simply unscrewing the plugs from the sections and looking into the filler necks. The normal liquid level in the section should be approximately 1 cm above the cut-off level of the plates. Some batteries even have a special mark stamped on the case. If the level is low, then the situation, although serious, can be easily eliminated. For this operation you will need:
- a medical syringe without a needle or a car hydrometer;
- distilled water;
- protective equipment (glasses and rubber gloves).
Distilled water is drawn into a syringe and poured into the appropriate sections to the required level. After adding liquid to the battery, it is placed on charge. In this regard, an autohydrometer is much preferable, since by adding water, you can immediately control the density of the solution.
Be careful not to work with acid unless your eyes are protected.
How to check battery density
It is recommended to check the density value after driving 15-20 thousand kilometers.
To do this, you need to buy a densimeter - this device is easy to purchase at car dealerships. It consists of a glass flask in the form of a tube tapering towards one end, at the other end of which there is a rubber bulb. A float-hydrometer is placed in the expanded part of the densimeter.
To carry out the measurement, you need to remove the plugs from the battery. The narrow end of the densimeter is inserted into the jar, immersed in the electrolyte, and the solution is drawn inside by squeezing the bulb.
Its quantity should be such that the hydrometer floats freely inside the densimeter. By combining the scales on the hydrometer and the densimeter flask, the exact density value is determined, which indicates the amount of charge of the battery.
It is not possible to check the density on all batteries. Now in passenger vehicles, many drivers prefer to buy maintenance-free models that do not have access to the inside of the cans. There are also so-called low-maintenance models, in which the only thing you can do is add distillate.
For such batteries, the density is determined using a special indicator located on the top cover. It is a colored window. If it is green, it means that the charge level is 65-100%, half charge or below is shown in black, white or red colors indicate the need to add water.
Battery charge level indicator.
You can always clarify the meaning of the color on the indicator on the battery label, it is always placed.
Attention! To find out whether it is necessary to adjust the electrolyte density, you need to check it only on a fully charged battery.
The first thing you need to do is check the level and add distilled water if necessary. Then it charges fully with the charger.
After turning off the charger, the battery should be allowed to stand for 2-3 hours so that the measurement data is as reliable as possible.
To avoid making mistakes, measure the air temperature in the room where charging was done with a thermometer and see what the density should be at that temperature in the table.
Now you can begin the verification process. Using a densimeter, draw up the electrolyte so that the hydrometer floats freely.
It should float calmly without touching the walls of the flask. Raise the device to eye level and take readings, writing them down immediately.
Measurements must be taken for each jar.
Checking the electrolyte density with a densimeter.
Refer to the following table for determining the battery charge level depending on its density:
| Room temperature, °C | Charge level, % | ||
| 100 | 70 | Discharged | |
| More than 25 | 1,21–1,23 | 1,17–1,19 | 1,05–1,07 |
| Less than 25 | 1,27–1,29 | 1,23–1,25 | 1,11–1,13 |
Attention! The density in different banks on the battery should not differ.
If in some banks the density is lower than in other cells, this means that it is defective and there is a short circuit between the plates.
If the indicator is low in all elements, then this indicates that the battery is completely discharged, or sulfation of the plates has occurred. It is also possible that the battery is simply old and has expired.
The exact cause can be determined by a thorough check, during which, in addition to density, the voltage at the battery outputs is also measured under load and without it.
Density versus voltage curve according to charge.
Too high a density in jars is also not a reason for joy. This may mean that the electrolyte boiled during charging. When it boils, the density increases.
You can measure the density of the electrolyte to find out the battery charge level without a densimeter and without removing the battery from its seat on the car.
To check, you only need a multimeter and the table below, which shows the relationship between voltage readings and density.
6 Best Automotive Multimeters
| Battery charge level, % | Electrolyte density value, g/cubic. cm | Terminal voltage, batteries V |
| 100 | 1.28 | 12.7 |
| 80 | 1.245 | 12.5 |
| 60 | 1.21 | 12.3 |
| 40 | 1.175 | 12.1 |
| 20 | 1.14 | 11.9 |
| 0 | 1.10 | 11.7 |
Attention! The density must be the same in all battery cells. The maximum deviation should not exceed 0.02–0.03 g/cubic meter. cm.
The voltage indicated in the table is valid for batteries kept at rest for at least 8 hours.
To restore battery performance that has decreased due to a drop in electrolyte concentration, it is necessary to adjust the density level.
To do this, part of the electrolyte is taken from the battery, and a correction solution having a density of 1.4 g/m3 is added instead. cm.
If the density is too high, then the selected electrolyte is replaced with distilled water.
Then the battery is charged for half an hour at the rated current, after which it is kept at rest for several hours so that the density of the electrolyte becomes the same in all banks.
Attention! When carrying out all work with electrolyte, extreme caution must be observed. There should be baking soda and a source of running water nearby. This will quickly neutralize the acid if it comes into contact with the skin. Inhaling vapors is also very harmful, so work is carried out only in a well-ventilated non-residential area.
What is a hydrometer
A device for measuring the concentration of various solutions is called a hydrometer. The operation of the density meter is implemented on the basis of a well-known physical law.
Archimedes' principle states that if a body is immersed in a liquid, a hydrostatic or lifting force will begin to act on it. In modulus it will be equal to the weight of the liquid that the body displaced, but opposite in direction.
For practical use, the hydrometer scale is graduated to the concentration of the dissolved substance. Car enthusiasts will find a useful device for checking the density of electrolyte and antifreeze.
How to increase the density in a battery
It is not so easy to correctly increase the density of the electrolyte in a battery, so we will dwell on this issue in detail.
They increase the density if, after repeated dilution with distillate, the concentration is no longer sufficient for normal battery operation in winter.
The procedure must also be carried out if the battery has been subjected to prolonged recharging several times. A decrease in the rate of the charge-discharge cycle indicates that it is time to adjust the composition of the electrolyte.
To increase density, use:
- correction electrolyte with a density of 1.40 g/cc. cm;
- concentrated sulfuric acid.
Attention! It is better for non-professionals not to use acid, as the slightest carelessness can lead to injury and even disability.
To work you will need:
- densimeter;
- sulfur cup with scale;
- rubber bulb or enema;
- correction electrolyte;
- container for diluting electrolyte;
- distilled water.
The algorithm for increasing the concentration of the solution looks like this:
- We take a small volume of electrolyte from the jar with a pear.
- In return, we return the same amount of correction solution.
- The battery needs to be recharged with a charger for 30 minutes for the liquid to mix.
- After the charging cycle, the battery is disconnected from the charger and left alone so that the electrolyte cools down, the density becomes uniform in all banks and bubbles come out. This is done for the accuracy of subsequent measurements.
- A control measurement is carried out to determine whether the adjustment cycle needs to be repeated.
Attention! If the density value in individual battery elements exceeds 0.01 g/cub. cm, then the battery must be charged again at a current value 2-3 times lower than the nominal one.
To calculate how much water or concentrated electrolyte to add to the battery, you need to know exactly its volume.
The composition of the electrolyte is approximately 40% sulfuric acid and 60% water.
To make it easier to change the concentration of the solution, use the following table:
Electrolyte concentration adjustment table.
If you need to make the density higher, add correction electrolyte, if lower, add distilled water.
The table is designed only for the use of concentrated electrolyte with a density of 1.40 g/cubic meter. cm, not acid.
Design and principle of operation of the battery
In order to properly service the battery and ensure its proper operation, it is necessary to at least approximately understand what is inside it and how it all works. Therefore, before moving on to questions about the electrolyte, it is necessary to understand how a car battery works and on what principle it works.
Battery design
Almost all lead-acid batteries have the same design. They consist of separate sections (cans), each of which has a set of positive and negative plates. The first are called cathode and are made of metal lead. The second, anode, are made of lead dioxide. The plates are collected in a bag and placed in an acid-resistant container, into which the working fluid is subsequently poured - an aqueous solution of sulfuric acid or the so-called electrolyte.
Lead-acid battery section design:
- 1 – jar lid;
- 2 – can body;
- 3 – ribbed settling tank;
- 4 – plates collected in a package;
- 5 – negative (anode) terminal;
- 6 – negative (anode) plates;
- 7 – dielectric gasket – separator;
- 8 – positive (cathode) terminal;
- 9 – positive (cathode) plates.
The finished sections connected in series are the battery. There are three such sections in six-volt batteries, and six in 12-volt batteries.
How it works
So, the design of the battery is quite simple, but how does voltage appear at its terminals? Indeed, if you take a battery straight from the store and connect a voltmeter to it, the device will show “0”. The lack of current is due to the fact that the electrolyte is not poured into the battery immediately after manufacture, and the plates in the battery standing on the store shelf are dry. The working fluid is poured into the battery after purchase.
It's time to find out what the electrolyte is for . Since the positive and negative plates have different chemical compositions, a potential difference arises between them when immersed in an acid solution (about 2 V per section, which determines the number of sections in the battery). When a load is connected to the battery terminals, current begins to flow between the plates due to the high electrical conductivity of the electrolyte. At the same time, the chemical process of converting lead dioxide into lead sulfate begins with the participation of sulfuric acid. As soon as the amount of dioxide and sulfuric acid drops to a certain level, the process will stop and the battery will stop producing current - it will be discharged.
During the discharge process, sulfuric acid and lead dioxide are consumed to form lead sulfate
We recommend: Banner car batteries with Austrian quality
But batteries, unlike galvanic cells (batteries), can restore their chemical properties. If you connect the battery to a DC source, then under its influence the sulfate will begin to decompose into lead dioxide and sulfuric acid. The battery will begin to charge, converting electrical energy into chemical energy. As soon as the amount of dioxide and acid reaches the initial values, the battery can be considered charged.
Chemical processes that occur in a battery when it is discharged and charged
Sulfuric acid, which is part of the electrolyte, plays one of the main roles in the operation of the battery. The quality and long-term operation of the battery as a whole will depend on its properties.