As part of regular car maintenance, one of the important operations is measuring the density of antifreeze. The article describes in detail why this should be done, what and how, what indicators should be, and how to adjust them if necessary. We also offer information on how to make antifreeze with your own hands by diluting the concentrate, and why it is profitable to do this.
WHY measure the density of antifreeze?
Antifreeze density is a characteristic of the coolant, which indicates the percentage of ethylene glycol content in the total measured volume. This indicator, in turn, determines the maximum temperature below zero, to which the antifreeze does not crystallize, that is, it does not freeze.
Antifreeze and antifreeze are the same thing. There is information that they have a different boiling point, but this is not true. As for the density considered here, the principle is absolutely the same.
Let us briefly examine how the freezing point of a coolant depends on density. If you take ordinary distilled water, then its density will be equal to 1, and the temperature at which it begins to freeze will be 0°C. If you dilute concentrated ethylene glycol with water in a 1:1 ratio, the density of the resulting liquid will already be 1.071, and it will begin to freeze at temperatures below -40°C.
One important feature to remember is the property of concentrated ethylene glycol to crystallize at a temperature of only -20°C. That is, in this case the principle does not work - the more ethylene glycol in the coolant, the lower its freezing point . This component effectively protects the cooling system from freezing only in certain proportions with water. Why this is so is quite difficult to explain in simple words, so just remember it as a fact.
Actually, for this reason there is a need to measure the density of antifreeze. At the same time, it doesn’t matter whether the liquid has already been poured into the car’s cooling system, or just purchased. In the first case, it may turn out that over time the density of the antifreeze has changed, and accordingly, the temperature at which freezing will occur has shifted. In the case of what is called “from a bottle” liquids, today you can easily run into a cheap (or maybe expensive) counterfeit, the density of which will be insufficient for use in your region.
HOW to measure the density of antifreeze?
To measure the density of antifreeze, there is a simple and inexpensive device - a hydrometer . It has exactly the same design as for measuring the density of electrolyte in batteries. The operating principle is similar. A transparent case with a scale contains a float, which, depending on the density of the collected liquid, floats more or less.
The difference between hydrometers for electrolyte and antifreeze is only in the range of measured densities. This is due to the fact that for coolant the normal average density is 1.07. But for the electrolyte – 1.26.
In addition, on hydrometers for measuring the density of antifreeze, an auxiliary scale can be applied, which can be used to clearly determine the freezing point of the liquid being tested. Similarly, battery devices have color grading, which allows you to understand, without delving into the topic, how many percent the battery is charged or discharged.
What to look for when taking measurements
It should be remembered that the accuracy of the hydrometer is influenced by certain conditions:
- Purity. The device and its components must be transparent and clean, especially the scale and bulb. Rinse the device thoroughly after each measurement.
- Temperature. For more accurate readings, the device and the liquid being measured must be at the same temperature (ideally 20 degrees).
- Design features. The flask should exceed the diameter of the hydrometer itself by 2 - 3 cm, so when taking liquid the meter will not touch the walls of the body and will give more accurate readings.
When purchasing, pay attention to the size of the scale; it should be as large and more accurate as possible. For electrolyte, 0.01 g/cm3 is better. When choosing the material from which the device is made, remember that low-quality plastic may become cloudy over time, but it is safe to use. Glass does not lose its transparency, but requires careful handling.
HOW to measure the density of antifreeze?
The principle of measuring electrolyte density is the same as the more familiar procedure with batteries. To obtain data, it is necessary to remove liquid from the expansion tank, radiator or storage container in a volume sufficient for the float inside to reliably float up. Then, based on the numbers printed on the float, determine either the density in the appropriate units or the freezing point in degrees Celsius.
In order for the obtained indicators to be as accurate as possible, one very important condition must be adhered to. It refers to the current temperature of the liquid being tested. The fact is that the scale printed on the device, as well as the numbers that can be found in special tables, correspond to reality only if the antifreeze temperature is at around 20°C.
For this reason, if you decide to measure the density of antifreeze poured into the cooling system, do this procedure when the engine is cool. For example, in the morning, or on a weekend when the car is not in use. The same applies to winter measurements. At negative ambient temperatures, the readings will also be inaccurate.
In fact, it is possible to measure the density of antifreeze at any temperature, however, to obtain accurate data it will be necessary to make adjustments using special formulas. It’s easy to get confused in them, so it’s much easier to choose the optimal conditions and simply measure the density with a hydrometer without any corrections.
WHAT should the density of antifreeze be?
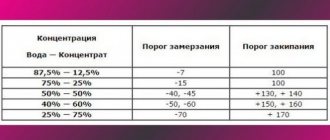
A little has already been said about this above. Let's repeat. If the density of the coolant is 1.071 , this means that it will not freeze at ambient temperatures down to -40°C . This density means that your antifreeze contains ethylene glycol and water in approximately equal proportions.
What about other density indicators? To navigate them, there are tables, the use of which, by and large, is not at all necessary. It is enough to know that with a density of 1.06, antifreeze will freeze at -30°C; if the hydrometer shows 1.05, then freezing will occur at -20°C . If the density is even lower - 1.035, then the cooling system will freeze at -10°C .
There is no need to be afraid of increasing the density in the other direction, since no manufacturer makes such an antifreeze that is “unprofitable” for itself. In a car, the density of the coolant cannot increase in any way either. It can only go down if the system is not sealed and you regularly add water to restore the required level. Water is also often added after repairs associated with disassembling cooling system components.
HOW to adjust the density of antifreeze?
As is clear from the above, adjustment of the density of purchased or used antifreeze is most often required in the direction of increase. Let's consider it. To make the adjustment, you will need a hydrometer, ethylene glycol concentrate, and knowledge of the volume of your vehicle's cooling system.
To make the process easier to understand, let's give a specific example. It will be possible to navigate by it. Let’s assume that our car is filled with antifreeze, the density of which turns out to be 1.05, which corresponds to a freezing temperature of -20°C. And in the region where it is operated, the temperature in winter drops to -29°C. The volume of the cooling system in this example is 7 liters.
Our task is to increase the density of 7 liters of antifreeze from 1.05 to 1.06 using a concentrate. The current density suggests that our liquid contains approximately 36% ethylene glycol (data from the tables), that is, 2.5 liters. And to achieve the desired density of 1.06, we need it to be 45%, or 3.1 liters. The rest is water. How to adjust?
And we just need to add 0.6 liters of ethylene glycol concentrate to the cooling system. This procedure is very simple. First, through the drain plug on the radiator, without unscrewing it completely, carefully drain 600 milliliters of coolant from the system. We tighten the plug and pour 600 milliliters of concentrate into the expansion tank or radiator. All. During operation of the cooling system, ethylene glycol will mix with the rest of the liquid, and the density will change to the desired values.
Similarly, you can increase the density with other indicators and volume of the system. In order, on the contrary, to reduce the density, using the same principle, we compensate for the drained liquid not with concentrate, but with distilled water.
How much does a 5 liter plastic bottle weigh?
Do you know how much one plastic bottle weighs on average? Let's take one of the most popular containers - 1.5 liters. Its weight will be 41 grams.
Interesting materials:
How to insulate a bathhouse from the inside with your own? How to insulate a door in a private house? How to clarify SNILS? How is the shift schedule approved? How to notify a tenant of termination of a lease? How to notify about a residence permit? How to increase the net assets of an enterprise? How to increase FPS in Pubg Mobile? How to enlarge a T-shirt? How to increase the volume through the Samsung engineering menu?
HOW to dilute antifreeze concentrate?
After reading the above material, there should no longer be any difficulties in preparing antifreeze of suitable density from ethylene glycol concentrate. Let's briefly consider why do this at all, if the market is full of ready-made compounds, on the label of which the main parameter for the car enthusiast is written - freezing temperature.
- Firstly, the characteristics indicated on the packaging do not always correspond to reality. Many manufacturers of budget coolants simply save money by not adhering to the proportions discussed above. Water is cheaper than ethylene glycol.
- Secondly, it is quite possible today to find ethylene glycol concentrate at a very competitive price. Naturally, it will be more expensive than ready-made compounds. But you will need almost twice as much of it. In addition, by preparing antifreeze with your own hands, you will know exactly what density it is and to what temperature it is guaranteed not to freeze.
In conclusion, let's look at a brief concrete example of how to dilute the concentrate. To do this, you will need, in fact, the concentrate itself, distilled water (required), a hydrometer to control the resulting density, and a couple of containers of the appropriate size.
How much to buy depends on how big your car's cooling system is and the coldest winter temperature in your area. Let's consider the situation using the example of the same car with a cooling system with a volume of 7 liters, which is operated in a region where the thermometer drops to a maximum of -29°C.
To prevent the prepared coolant from freezing in winter, we need to obtain antifreeze with a density of 1.063. This figure corresponds to 45.6% ethylene glycol content in the total volume. Accordingly, to prepare 7 liters of antifreeze we need 3.2 liters of concentrate and 3.8 liters of distilled water. Mix, mix thoroughly, measure the density with a hydrometer and pour into the cooling system.
Verification methods
There are several options for checking the density of antifreeze with your own hands. Moreover, such procedures are easy to perform at home or in the garage.
Before measuring the current density of antifreeze, you should decide on the method used. Each of them requires the presence of certain devices.
To check the current density of antifreeze at home, you can use:
- hydrometer;
- refractometer;
- folk methods.
With such measurements of the density of antifreeze, the motorist will find out the current state of the liquid and decide on further actions with it.
Hydrometer
The easiest way to check the current density of filled or purchased antifreeze is with a hydrometer.
This is an affordable measuring device that clearly reflects the density values of automotive antifreeze. You can purchase it at any auto parts store. The purchase will cost an extremely modest amount.