The MPa to Bar or Atm pressure converter is a tool that will free you from the need to independently calculate the pressure indicator in the unit of measurement you need. All you need to do to calculate is to enter the required numbers. The service will instantly produce an error-free result.
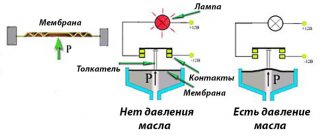
It will be useful for car owners who need to quickly calculate how much a specific MPa number will be in Atm or Bar. The data obtained can be useful in assessing tire pressure and analyzing the condition of the fuel supply system.
You can find out the MPa indicator in tires using a pressure gauge.
The pressure calculator is also useful for car owners when refilling their car’s air conditioner with freon.
When converting pressure units, rely on the following relationships:
| MPa to Bar | 1 MPa = 10 bar |
| MPa to Atm | 1 MPa = 9.869 atm |
| Psi to Bar | 1 Psi = 0.069 bar |
| Psi to Atm | 1 Psi = 0.068 bar |
| Bar to MPa | 1 Bar = 0.1 MPa |
There are some nuances when rounding. An integer up to 15 characters will be considered accurate. There must be no more than 10 digits after the decimal point.
| Meaning | MPa | Bar | atm | kgf/cm2 | psi | at |
| 1 MPa | 1 | 10 | 9,8692 | 10,197 | 145,04 | 10.19716 |
| 1 bar | 0,1 | 1 | 0,9869 | 1,0197 | 14,504 | 1.019716 |
| 1 atm (physical atmosphere) | 0,10133 | 1,0133 | 1 | 1,0333 | 14,696 | 1.033227 |
| 1 kgf/cm2 | 0,098066 | 0,98066 | 0,96784 | 1 | 14,223 | 1 |
| 1 PSI (lb/in²) | 0,006894 | 0,06894 | 0,068045 | 0,070307 | 1 | 0.070308 |
| 1 at (technical atmosphere) | 0.098066 | 0.980665 | 0.96784 | 1 | 14.223 | 1 |
General information
Balloon bursting in the TranslatorsCafe.com office
In physics, pressure is defined as the force acting on a unit surface area. If two equal forces act on one larger and one smaller surface, then the pressure on the smaller surface will be greater. Agree, it is much worse if someone who wears stilettos steps on your foot than someone who wears sneakers. For example, if you press the blade of a sharp knife onto a tomato or carrot, the vegetable will be cut in half. The surface area of the blade in contact with the vegetable is small, so the pressure is high enough to cut that vegetable. If you press with the same force on a tomato or carrot with a dull knife, then most likely the vegetable will not cut, since the surface area of the knife is now larger, which means the pressure is less.
In the SI system, pressure is measured in pascals, or newtons per square meter.
How to use an online calculator?
Pressure in a coffee maker: how much bar should it be
Description of the calculator functionality
- Left column of the calculator. Contains a selection of initial values. A precise technical description of the quantity is displayed below each column.
- Right column of the calculator. Contains the final translation value. Under each column there is a detailed description of the final transfer amount.
- Getting results. To convert MPa to pascals, enter the values of the original value. The online calculator will quickly translate the original data.
To convert extremely large and small numbers, a separate concept is used: computer exponential notation. Using this method, it is possible to write numbers with high concomitant abbreviations.
Who is the online calculator designed for?
- For specialists who conduct scientific research. You can easily convert, for example, a bar to a torr.
- For vehicle owners. Very often it is necessary, for example, to convert MPa to bars. This data is used to analyze the condition of the fuel line, as well as to check the nominal tire pressure of the vehicle.
- Car owners use a calculator when converting a single value into MPa in the process of filling car parts with freon.
Atmosphere pressure
Atmospheric pressure is the air pressure at a given location. It usually refers to the pressure of a column of air per unit surface area. Changes in atmospheric pressure affect weather and air temperature. People and animals suffer from severe pressure changes. Low blood pressure causes problems of varying severity in humans and animals, from mental and physical discomfort to fatal diseases. For this reason, aircraft cabins are maintained above atmospheric pressure at a given altitude because the atmospheric pressure at cruising altitude is too low.
The aneroid contains a sensor - a cylindrical corrugated box (bellows) connected to an arrow, which rotates when the pressure increases or decreases and, accordingly, the bellows compresses or expands
Atmospheric pressure decreases with altitude. People and animals living high in the mountains, such as the Himalayas, adapt to such conditions. Travelers, on the other hand, should take the necessary precautions to avoid getting sick due to the fact that the body is not used to such low pressure. Climbers, for example, can suffer from altitude sickness, which is associated with a lack of oxygen in the blood and oxygen starvation of the body. This disease is especially dangerous if you stay in the mountains for a long time. Exacerbation of altitude sickness leads to serious complications such as acute mountain sickness, high altitude pulmonary edema, high altitude cerebral edema and extreme mountain sickness. The danger of altitude and mountain sickness begins at an altitude of 2400 meters above sea level. To avoid altitude sickness, doctors advise not to use depressants such as alcohol and sleeping pills, drink plenty of fluids, and rise to altitude gradually, for example, on foot rather than by transport. It's also good to eat plenty of carbohydrates and get plenty of rest, especially if you're going uphill quickly. These measures will allow the body to get used to the oxygen deficiency caused by low atmospheric pressure. If you follow these recommendations, your body will be able to produce more red blood cells to transport oxygen to the brain and internal organs. To do this, the body will increase the pulse and breathing rate.
First medical aid in such cases is provided immediately. It is important to move the patient to a lower altitude where the atmospheric pressure is higher, preferably to an altitude lower than 2400 meters above sea level. Medicines and portable hyperbaric chambers are also used. These are lightweight, portable chambers that can be pressurized using a foot pump. A patient with altitude sickness is placed in a chamber in which the pressure corresponding to a lower altitude is maintained. Such a chamber is used only for providing first aid, after which the patient must be lowered below.
Some athletes use low pressure to improve circulation. Typically, this requires training to take place under normal conditions, and these athletes sleep in a low-pressure environment. Thus, their body gets used to high altitude conditions and begins to produce more red blood cells, which, in turn, increases the amount of oxygen in the blood, and allows them to achieve better results in sports. For this purpose, special tents are produced, the pressure in which is regulated. Some athletes even change the pressure in the entire bedroom, but sealing the bedroom is an expensive process.
Correlation and conversion of quantities
The need to convert units of measurement from different metric systems is in demand in production. Equipment supplied by manufacturers, especially foreign ones, is often equipped with control devices with unusual calibration. To quickly make a translation, you need to know that:
- The difference between technical and standard atmospheres is small (1 atm = 1.033 atm), so it is neglected in practical calculations.
- One megapascal is equal to 10.1972 kgf/cm² or 9.8692 atm.
- The technical atmosphere in MPa for technical calculations is considered equal to 0.1, and the physical atmosphere is 0.987.
- 1 mm water Art. with sufficient accuracy can be taken equal to 10 Pa, for mercury 133 Pa.
- Using the specified values in calculations gives an error of 0.5%. A result with an error of 2% is obtained if the initial data is based on the calculation of 1 MPa = 10 at. The ratio 1 MPa = 10 atm is taken when an error of 3% is permissible when converting atmospheric pressure.
To convert megapascals to atmospheres, coefficients are used by which the original value is divided. To get the result in atm 0.101972, for atm 0.098692.
To find out how many technical atmospheres contain 12 MPa, you need to enter 12/0.101972 into the search bar, for example in Yandex. After instant calculation, the result will be 117.679362962.
Spacesuits
NASA's reusable transport spacecraft Atlantis is on display at the Kennedy Space Center.
Pilots and astronauts have to work in low-pressure environments, so they wear spacesuits that compensate for the low pressure environment. Space suits completely protect a person from the environment. They are used in space. Altitude-compensation suits are used by pilots at high altitudes to help the pilot breathe and counteract low barometric pressure.
Pressure drop
When water flows through a pipe, the pressure at the outlet will be less than at the inlet.
The fall is determined by several factors:
- Pipe diameter.
- Its length.
- The roughness of her walls.
Plastic water pipes have much smoother walls than any metal ones.
- The speed of the flow in it.
The formula used for calculation is H = iL(1+K).
In it:
- H – pressure drop in meters. To convert it to atmospheres, it is enough to divide the resulting value by 10.
- i is the hydraulic slope, determined by the diameter, material of the pipe and the flow rate in it.
- L is the length of the pipe in meters.
- K – coefficient, for domestic and drinking water supply systems, taken equal to 0.3.
Where can I get the hydraulic slope value? In the so-called Shevelev tables. Here is a fragment of one of them, relevant for a new steel pipe of size DN15.
| Water consumption, l/s | Flow speed, m/s | 1000i |
| 0,17 | 1,00 | 266,2 |
| 0,18 | 1,06 | 296,1 |
| 0,19 | 1,12 | 327,6 |
| 0,20 | 1,18 | 360,5 |
| 0,25 | 1,47 | 560,4 |
| 0,30 | 1,77 | 807,0 |
| 0,35 | 2,06 | 1098 |
The value 1000i is the hydraulic slope for a pipe length of 1 km. To calculate the value of i for a linear meter, it is enough to divide it by 1000.
So, for a DN15 steel pipe 25 meters long with a water flow through it of 0.2 l/s, the pressure drop will be (360.5/1000)*25*(1+0.3)=11.7 meters, which corresponds to the difference pressure of 1.17 kgf/cm2.
The hydraulic slope values given are valid for new pipes. Over time, lime and rust will increase their hydraulic resistance and reduce clearance.
Hydrostatic pressure
Hydrostatic pressure is the pressure of a fluid caused by gravity. This phenomenon plays a huge role not only in technology and physics, but also in medicine. For example, blood pressure is the hydrostatic pressure of blood on the walls of blood vessels. Blood pressure is the pressure in the arteries. It is represented by two values: systolic, or the highest pressure, and diastolic, or the lowest pressure during a heartbeat. Devices for measuring blood pressure are called sphygmomanometers or tonometers. The unit of blood pressure is millimeters of mercury.
Digital blood pressure machine, also called a sphygmomanometer
The Pythagorean mug is an interesting vessel that uses hydrostatic pressure, specifically the siphon principle. According to legend, Pythagoras invented this cup to control the amount of wine he drank. According to other sources, this cup was supposed to control the amount of water drunk during a drought. Inside the mug there is a curved U-shaped tube hidden under the dome. One end of the tube is longer and ends in a hole in the stem of the mug. The other, shorter end is connected by a hole to the inside bottom of the mug so that the water in the cup fills the tube. The principle of operation of the mug is similar to the operation of a modern toilet cistern. If the liquid level rises above the level of the tube, the liquid flows into the second half of the tube and flows out due to hydrostatic pressure. If the level, on the contrary, is lower, then you can safely use the mug.
What is PSI
PSI, the most well-known for American countries, is naturally indicated in many instructions for models produced by manufacturing companies in this country. The pressure value of one pound per inch squared is what this unit of measurement means.
That is why it is so important to understand and be able to quickly translate the values of PSI units, which are indicated in the technical documentation for a specific car brand, into understandable and convenient settings.
Pressure in geology
Quartz crystal illuminated by laser pointer
Pressure is an important concept in geology. Without pressure, the formation of gemstones, both natural and artificial, is impossible. High pressure and high temperature are also necessary for the formation of oil from the remains of plants and animals. Unlike gems, which primarily form in rocks, oil forms at the bottom of rivers, lakes, or seas. Over time, more and more sand accumulates over these remains. The weight of water and sand presses on the remains of animal and plant organisms. Over time, this organic material sinks deeper and deeper into the earth, reaching several kilometers below the earth's surface. The temperature increases by 25 °C for every kilometer below the earth's surface, so at a depth of several kilometers the temperature reaches 50–80 °C. Depending on the temperature and temperature difference in the formation environment, natural gas may form instead of oil.
Diamond tools
Natural gemstones
The formation of gemstones is not always the same, but pressure is one of the main components of the process. For example, diamonds are formed in the Earth's mantle, under conditions of high pressure and high temperature. During volcanic eruptions, diamonds move to the upper layers of the Earth's surface thanks to magma. Some diamonds fall to Earth from meteorites, and scientists believe they formed on planets similar to Earth.
Synthetic gemstones
The production of synthetic gemstones began in the 1950s and has been gaining popularity recently. Some buyers prefer natural gemstones, but artificial stones are becoming more and more popular due to their low price and lack of hassles associated with mining natural gemstones. Thus, many buyers choose synthetic gemstones because their extraction and sale is not associated with human rights violations, child labor and the financing of wars and armed conflicts.
One of the technologies for growing diamonds in laboratory conditions is the method of growing crystals at high pressure and high temperature. In special devices, carbon is heated to 1000 °C and subjected to pressure of about 5 gigapascals. Typically, a small diamond is used as the seed crystal, and graphite is used for the carbon base. From it a new diamond grows. This is the most common method of growing diamonds, especially as gemstones, due to its low cost. The properties of diamonds grown in this way are the same or better than those of natural stones. The quality of synthetic diamonds depends on the method used to grow them. Compared to natural diamonds, which are often clear, most man-made diamonds are colored.
Due to their hardness, diamonds are widely used in manufacturing. In addition, their high thermal conductivity, optical properties and resistance to alkalis and acids are valued. Cutting tools are often coated with diamond dust, which is also used in abrasives and materials. Most of the diamonds in production are of artificial origin due to the low price and because the demand for such diamonds exceeds the ability to mine them in nature.
Some companies offer services for creating memorial diamonds from the ashes of the deceased. To do this, after cremation, the ashes are refined until carbon is obtained, and then a diamond is grown from it. Manufacturers advertise these diamonds as mementos of the departed, and their services are popular, especially in countries with large percentages of wealthy citizens, such as the United States and Japan.
Story
It was originally defined as a pressure exerted by 760 mmHg. Art. At 0 °C and standard gravity ( g
n =9.806 65 m/s 2 ).
It was used as a reference condition for physical and chemical properties and was implied in the definition of the Celsius (later Celsius) temperature scale, defining 100 °C as the boiling point of water at that pressure. In 1954, the 10th General Conference on Weights and Measures (CGPM) adopted a standard atmosphere
for general use and confirmed its definition: 1,013,250 dynes per square centimeter (101,325 Pa). This determined both temperature and pressure, regardless of the properties of a particular substance. In addition (noted by the CGPM) there was some misconception that this "has led some physicists to believe that this definition of a standard atmosphere is valid only for precise work in thermometry."
In chemistry and various industries, the reference pressure specified in "standard temperature and pressure" (STP) was usually 1 atm (101.325 kPa), but standards have diverged since then; in 1982, the International Union of Pure and Applied Chemistry (IUPAC) recommended that, for the purpose of determining the physical properties of substances, " standard pressure
"was exactly 100 kPa (1 bar).
Calculation of the recommended value
How to use the calculator?
The calculator is working in test mode! If you find an error in the indicators or want to leave suggestions, please send them through the feedback form on the Feedback page or the VK group! Errors with evidence (photos, screenshots, links) are accepted! All information presented on the site is for informational purposes only! Thanks for understanding!
Contents
Watch water resistance standards
There are many different standards that determine the water resistance of watches and other electronic devices (such as phones). Waterproof watches are very popular among tourists, climbers and extreme sports enthusiasts.
Watch water resistance standard ISO 2281 (GOST 29330)
This standard was adopted in 1990 to standardize the water resistance of watches. It describes the procedure for checking the water resistance of a watch during testing. The standard specifies the requirements for water or air pressure at which the watch must maintain its tightness and performance. However, the standard states that it can be carried out selectively. This means that not all watches produced according to this standard undergo mandatory water resistance testing - the manufacturer can selectively check individual pieces. This standard is used for watches that are not specifically designed for diving or swimming, but only for watches for daily use with possible short-term immersion in water.
Testing a watch to this water resistance standard consists of the following steps:
- Immerse the watch in water to a depth of 10 cm for one hour.
- Immerse the watch in water to a depth of 10 cm with a water pressure of 5 N (newtons) perpendicular to the buttons or to the crown for 10 minutes.
- Immerse the watch in water to a depth of 10 cm and change the temperature between 40°C, 20°C and again 40°C. The watch stays at each temperature for five minutes, the transition between temperatures is no more than five minutes.
- Immersion of watches in water in a pressure chamber and exposure of them to their nominal pressure for which they are designed for 1 hour. Condensation inside the watch and water penetration into the case are not allowed.
- Checking the watch with 2 atm above the rated pressure.
Well, additional checks not directly related to the water resistance of the watch:
- The watch must not show streamlining exceeding 50 μg/min
- No strap test required
- No corrosion test required
- No negative pressure test required
- No magnetic field or shock test required
What are the dangers of driving on improperly inflated tires?
Many novice motorists have the misconception that an overinflated tire is preferable to an underinflated one. An argument for this can be that on hypercars the tire pressure is usually higher than normal. In fact, any pressure deviation from normal values has an equally negative effect on the tires. Thus, with insufficient pressure, the contact area of the tread with the road surface increases, which causes greater wear at the edges.
Insufficient tire pressure is indicated by:
- increased fuel consumption by 10-20%;
- cornering with great effort;
- the car moves to the side when driving at speeds above 50 km/h.
When the normal pressure value is exceeded, the tread has a smaller contact area with the blade, therefore increased wear is observed in the center. Tire pressure that is too high is indicated by:
- loss of control when cornering;
- car jumps at speeds above 60 km/h (if the wheel hits a pothole or runs over a rock, the tire may be damaged);
- suspension operation in overloaded mode;
- increased noise in the cabin.
When the vehicle is at maximum load, do not over-inflate the wheels. Some car enthusiasts do this, mistakenly believing that the load needs to be compensated by pumping.