If the coolant level has dropped slightly below the minimum mark, and there is no antifreeze on hand, is it possible to add water to the system? Can. But it must be borne in mind that the water must be distilled, and a certain volume of it can significantly increase the risk of freezing in winter. All other statements and advice from “experts” that can be found on the Internet are nothing more than unsubstantiated myths.
The article examines in detail each of the most common fictions in this regard. It also clearly explains how to check the quality of distilled water at home, and how much maximum it can be safely added to antifreeze in each specific case.
Is it necessary to dilute the coolant?
In some cases, adding purified water to the antifreeze solution is necessary.
This is advisable when the composition has become too concentrated. This occurs in the summer when the weather is hot.
Moisture evaporates rapidly. The solution becomes saturated. It can be diluted with a certain amount of distilled composition.
You can also dilute the composition with purified water if it has not been added to it previously. The distillate will not harm the new solution.
Do not add a lot of purified water to the coolant in winter. This is fraught with a violation of the consistency of the mixture, due to which it can quickly freeze even with a slight minus outside.
As a result, the radiator may rupture due to the solution freezing. Also, frozen antifreeze often leads to pipe ruptures.
Under what circumstances is it acceptable to dilute?
Despite numerous prohibitions and restrictions, car owners sometimes have to violate them. After all, if the refrigerant has partially leaked out, then what happens if antifreeze is mixed with water is not very important. It is better to take the risk than to continue driving with insufficient quantities of this chemical. However, there is a “golden” rule that should be strictly adhered to: the volume of added distilled water cannot exceed the volume of the remaining coolant. Alas, sometimes the driver simply has no choice - when the refrigerant has leaked or evaporated completely, and in order to get to the nearest auto shop or repair shop, he has to “drive” on water alone. However, you should move at minimum speed, otherwise the engine will quickly overheat.
How to check the quality?
There are two ways to check the quality of the distilled composition:
Boiling .
You need to pour some distilled water into a stainless steel container and put it on fire. We must wait until it boils completely. If there is sediment or traces at the bottom of the container, then the distillate is of poor quality. It should not be mixed with antifreeze.- Testing the solution for its ability to conduct current. You need to take the socket together with the light bulb and connect them to the socket plug with one wire.
The second wire must be broken and a pair of wire pieces screwed to its two ends. They will act as electrodes. These electrodes must be dipped into a glass of distilled water at a sufficient distance from each other. The plug must be inserted into the socket. Water purified from impurities will not conduct electric current. The light will not light up. If it lights up, then the water in the glass is not distilled.
Attention! Untested liquid must not be mixed. This will further negatively affect the condition of the entire car.
How long can you move on “water alone”?
As already mentioned, diluting antifreeze with water is only a last resort when there is simply no other way to get to the service station. Accordingly, water should be removed as soon as possible, because it cannot completely replace the factory refrigerant.
How long it is permissible to ride “on water” depends on many factors. Including the technical condition of the car, mileage and even the year of manufacture of the vehicle. However, as practice shows, you can move for a couple of hours without negative consequences. And if the engine is practically new or has been overhauled, then much more. And yet, you can’t thoughtlessly risk your car, otherwise subsequent repairs can cost quite a lot of money.
How to dilute it correctly?
You cannot mix such a liquid with purified water without first checking the level of the former in the tank. The distilled solution can only be added in a certain proportion.
Typically, about 300-400 ml of purified water added to the antifreeze mixture does not harm the cooling system. But the proportion of distillate may be more or less depending on the amount of composition in the tank at a particular moment.
If the car’s cooling system holds 8 liters of antifreeze composition, then approximately this liquid should contain equal parts purified water and ethylene glycol. 4 liters of two components.
If, when checking the system, a shortage of a whole liter of antifreeze is revealed, then the concentration of both components will be 3.5 liters.
In this case, it is recommended to dilute everything with 1 liter of distillate. As a result, the tank will contain 3.5 liters of ethylene glycol concentrate and 4.5 liters of purified water.
The ideal proportion should be 50% of each component.
In this example it will be like this:
- 8 liters – 100%
- 3.5 liters – x%
It is necessary to calculate the required percentage of ethylene glycol. X% = 3.5 * 100% / 8 = 43.75%. Distilled water in such a mixture will be 56.25% (100% - 43.75%). This is almost the perfect proportion.
Myths about adding water to antifreeze
As a result of a quick study of several Internet resources, I found as many as 7 untrue tips and statements regarding adding water to antifreeze. Intuitively, of course, all this is very easy to believe. And people believe, defending this or that deliberately false information on forums and social networks and applying it in life. But if you look at these recommendations, based on the real physics of the processes, the composition of antifreeze and facts, you can easily be convinced of their mythical nature.
Myth No. 1. Just the added water will freeze in winter
In the understanding of the “experts” who say this, water added to antifreeze will continue to “walk” through the cooling system in the form of a kind of plug or individual drops. Naturally, if you think like this, you can easily come to the conclusion that pure water will certainly freeze as soon as the ambient temperature drops below 0°C.
And this opinion is a myth because the added water will mix with antifreeze. That is, the ethylene glycol present in the system will dissolve in this water, and the entire coolant will become homogeneous. Yes - its properties will change. Yes – the freezing and boiling points will shift. But will the added water freeze? No.
Myth No. 2. Added water “down there” will make everything rusty.
All the parts seem to be made of metal. And metal, as you know, rusts from water. The myth is based on these concepts. However, if you add water to the antifreeze, no rust or corrosion will appear inside the cooling system. Moreover, even if you completely drain the antifreeze and fill the system with clean (distilled) water, there will be no corrosion on the scale in which it is “painted” in this case.
There are several explanations for this. Firstly, the parts of the cooling system are made of non-ferrous metals, which are not so easy to rust from ordinary water. Secondly, corrosion is an oxidation process. And for this you need oxygen. But the cooling system of a car is a sealed environment, the access of oxygen to which is limited.
Among other things, we should not forget that previously motorists did not even know what antifreeze was. The cooling systems were filled exclusively with water. Yes - it had to be drained at night in winter. But there was no such thing that the engine rotted from the inside due to water. These are fairy tales.
Myth No. 3. Instead of distilled water, you can add boiled water
The same is said about filtered water, settled and treated with ionizers. This is only recommended by those who do not fully understand why it is important to pour distilled water into the car’s cooling system, and no other. It's all about the salts. In those same salts that are deposited in the form of scale as a poorly soluble coating on the inner walls of the cooling system.
So, no filters, ionizers or boiling can completely purify water from these same salts. But the technology used to make distilled water can. During the distillation process, the purest vapor without impurities is obtained (they do not evaporate), which is then sharply cooled and turns into a liquid state.
If you simply boil the water, then only part of the salts contained in it may have time to settle on the walls of the dish in the form of scale. The bulk will remain and will gradually be deposited on the walls of the car’s cooling system. Well, trying to purify water from salts using filters and ionizers is completely nonsense.
Myth No. 4. Older cars can withstand adding water better
The myth is based on what was already mentioned above - previously all cars, or rather their engines, were cooled exclusively with water. And modern engines are filled with antifreeze. Accordingly, some people have the impression that new cars are somehow not designed to handle water, and it will inevitably lead to disaster.
This is actually not true. Modern cooling systems are not much different from those of twenty years ago. At least in terms of chemical interaction with water. It’s just that antifreeze didn’t exist in nature before, and when it appeared, not everyone had the money for it. Today it is inexpensive, and compared to water it has a huge advantage when operating a car in winter. That's all.
Therefore, if your car is a very recent model, this does not mean that you absolutely cannot add a little water to the antifreeze if absolutely necessary. Can. But, again, observing the two conditions mentioned at the beginning of the article, and described in more detail below.
Myth No. 5. When adding water, the cooling capacity of antifreeze decreases sharply.
This is true, but not entirely. Ethylene glycol actually absorbs heat faster than water. Accordingly, if its concentration in antifreeze is reduced, it will remove heat from the engine more slowly. There are no questions here.
The only question is how slower the heat extraction will be, and whether this has any significance for the cooling system of the machine. So here it is. Even pure water is capable of removing heat slower than pure ethylene glycol so slightly that when adding it to antifreeze, the difference will be difficult to measure even with laboratory equipment. Moreover, the car’s cooling system is designed in such a way that it absolutely does not care what the rate of heat removal from the liquid is.
If the rate of heat extraction changes, the thermostat will simply open a little later, and the radiator fan will not work longer in the traffic jam. And so slightly later that you won’t even notice it. The engine, by the way, will also not be particularly upset.
Myth No. 6. Because of the water, the boiling point of the antifreeze will decrease and the system may rupture
This is partly true. The boiling point of pure ethylene glycol is about 200°C. Water temperature is 100°C. Antifreeze is nothing more than a mixture of these two substances in a ratio of approximately 50 to 50. Accordingly, its boiling point is something between the indicated two figures. But there are a couple of “buts”.
Firstly, when adding a small volume of water to antifreeze, the above ratio will hardly change (calculations with a specific example are below). Secondly, even if there is clean water in the cooling system, its boiling point is far from the 100°C we are used to. The fact is that as pressure increases, this figure increases. And since the cooling system is closed, the pressure in it increases noticeably as it heats up (this is easy to check if you carefully loosen the expansion tank cap on a warm engine).
The result is that the boiling point of antifreeze with added water will actually decrease. But it’s so insignificant that it won’t affect the operation of the system in any way, and certainly won’t break anything there.
Myth No. 7. After adding water, the antifreeze must be drained from the system as soon as possible.
This myth is based on all the previous ones. That is, if you add water to the antifreeze, then the coolant:
- will freeze;
- will cause corrosion;
- will “kill” a modern engine;
- it will cool worse;
- will boil and rupture the pipes.
As has already been proven, this is all untrue. But, again, only if two conditions are met - the water must be distilled, and its volume fraction in the antifreeze must also be observed. How to comply with these two conditions is described below.
Consequences of non-compliance with proportions
If water has been added in excess of the norm, then in winter it may freeze at a higher temperature than at the one that was originally set. The result may be damage by ice not only to the pipes, but also to the system tank itself. The engine radiator will be severely damaged.
If you do not add enough water, the system may work normally for some time. But inevitably there will be a lot of ethylene glycol in the composition. The mixture will become too concentrated. The mechanism will quickly overheat and fail.
It is possible to understand that the proportion of two components in the cooling system is disturbed only with the help of a hydrometer.
This device measures the density of liquids. The hydrometer is immersed in the composition.
Using its scale, the density of ethylene glycol and distillate in a non-freezing solution is calculated.
Where does the antifreeze go?
As you can see, the antifreeze already contains water, albeit distilled, from here you can immediately understand that adding water to the system at a low level is theoretically quite possible.
But at the same time, it is important to know how to do everything correctly so that there are no negative impacts from this.
A decrease in antifreeze level usually occurs in two cases:
- Evaporation (even though the water is distilled, it still changes its state of aggregation when exposed to high temperatures);
- Violation of the tightness of the system (loose clamp of the pipes, cracks in them and in the radiator).
In the first case, the loss of antifreeze is relatively insignificant, but in the second case, almost all of the liquid can leak out of the system if the leak is not noticed and corrected in time.
This is the first factor to consider when replenishing your level.
The second is weather conditions. It was noted above that a violation of proportions leads to a decrease in the temperature threshold for freezing of liquid.
What can be replaced?
No other liquid is suitable for diluting antifreeze . Neither tap nor boiled water is suitable for this. Even in water purified by a regular filter, salts and impurities still remain.
When they are added, the mixture loses its anti-corrosion properties. Due to poor quality water, all car systems will quickly fail.
Attention! Melt water and rainfall should not be used instead of distillate. They still contain foreign impurities.
This situation can happen to any driver
A car enthusiast faces many troubles during the operation of a car, including coolant leaks from the cooling system.
This phenomenon is quite common, but it often happens that the level of antifreeze in the cooling system needs to be replenished, but the necessary liquid is not available.
Otherwise, there is a high probability of overheating of the power plant.
So is it possible to add water to antifreeze or is it better not to do this? Let's figure it out.
Can distillate completely replace coolant?
Purified water cannot replace antifreeze. It is only an addition to the cooling mixture.
In summer, antifreeze can be diluted with water only if the solution itself becomes too concentrated.
In winter, the use of a cooling compound is necessary. Its dilution with distilled water during this period is not recommended, since a large amount of it can lead to freezing of the entire mixture.
Important! Complete replacement of antifreeze with distillate is fraught with rapid breakdown of the cooling system at any time of the year.
Level restoration with water
So, it is noted that the fluid level in the tank has dropped. At the same time, an inspection of the power plant and cooling system elements showed that there were no leaks. This means that the water has simply evaporated.
At the same time, the concentration of ethylene glycol (propylene glycol), as well as the additives present in the liquid, increased, since only water evaporated.
In this case, the distillate will have virtually no effect on the properties of the liquid. That is, you can safely add water, but only distilled water, bringing it to the required level.
In the case when a lot of fluid has been lost (there was a leak due to depressurization of the system), then the best option would be to top up with new antifreeze instead of leaking. But at the same time, be sure to fill only the liquid that was filled.
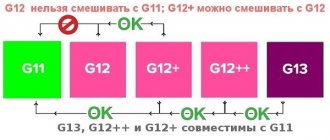
Antifreeze of a different brand and color should never be filled in. And all because each manufacturer uses its own additive packages, and there is a very high probability of conflict between them with a variety of negative consequences.
If you don’t have exactly the same antifreeze on hand, then filling the system with water is also allowed. The presence of water will not affect the operation of the power plant; the water will also maintain the temperature regime.
But there is also a negative point, which lies in the fact that there are no protective additives in the water, so it is impossible to predict how the effect of a pure distillate (or a weakly concentrated solution consisting of water and a small amount of residual antifreeze) will affect the components of the cooling system.
On some cars this will not have any negative consequences, but on others the process of corrosion of metal surfaces may begin.
Therefore, it is still possible to fill the system with a large amount of water, but you should reduce the time it is used as much as possible and replace it with antifreeze at the first opportunity in order to avoid negative consequences.
Winter time
During this period, adding water is strictly prohibited, even in small quantities. As a result, starting the engine at -5 degrees or more will be difficult. Frozen water due to expansion can rupture the radiator, pipes and tank. According to the requirements, the freezing point of antifreeze must be at least -25 degrees. With every milliliter of water added, this figure decreases.
Why only distilled?
Please note that tap water is absolutely not suitable for diluting coolant. It contains a large percentage of impurities that not only impair the ability of antifreeze to remove heat. The wear of the pipes also increases, corrosion of metal elements occurs, and salts become clogged in the radiator. Over time, it will simply clog in one of the channels and cause the engine to overheat.
To avoid engine problems, don't skimp on distilled water. But if you really need to go urgently, and you don’t have either one or the other at hand, you can add water, but only boiled water. And after such mixing, be sure to drain the antifreeze completely and pour in a new one. You can’t ride “on water” for a long time. Red antifreeze can be used as a new fluid. Its price is about 300 rubles for a five-liter canister. It is quite enough for a passenger car. But for minibuses and GAZelle-type trucks you need 10 liters. But still, this price is not comparable to the repair of the motor, which you will need in case of overheating.
About flowers
Green and red antifreeze are very popular now. Their prices are approximately the same. But there are differences. Green has several components:
- Organic substances.
- Inorganic.
- Chemical additives. They are borates, phosphates and carboxylic acid.
The advantage of using green antifreeze is the composition’s high resistance to corrosion. The mixture seems to “envelop” the insides with a protective film, preventing processes that are destructive to the metal. But there is also a minus here.
This film reduces the heat dissipation of the liquid. But this does not have a detrimental effect on the engine. If used correctly, it is very difficult for an engine to boil on green antifreeze. As for red antifreeze, their composition excludes the use of inorganic components. There is also a high percentage of carboxylic acid content. It does not form films inside the system, hence better heat transfer.
They also have an increased service life. High-quality red antifreeze lasts up to 5 years. However, the mixture has its drawbacks. This is primarily poor protection of aluminum radiators from scale. But if you have a copper or brass radiator, red antifreeze is the perfect choice for you. It is also sold in 5-liter cans. One of the popular ones. Reviews from motorists note a low freezing threshold - up to minus 35 degrees Celsius. The liquid does not boil at 110, but still you should not bring the engine to this state. This is very harmful for him.