The battery is an important component of any modern car. With its help, the engine starts, plus you can turn on different consumers without starting the engine. Thus, the power comes not from the generator, but from the battery.
But over time, the battery capacity may decrease. And at some point, when you turn the key in the ignition, nothing happens. Yes, car service specialists will say that the battery has reached its end, its service life has expired, and it is better to buy a new one. And they will probably tell you where and which one to buy.
But you shouldn't make hasty conclusions. A procedure such as CTC often allows you to resuscitate the battery. This is not a 100% guarantee of returning to full functionality, but a great way to save on buying a new battery. It's at least worth a try.
Why does the charge drop?
All batteries have a certain capacity, stated in Ah. In passenger cars, batteries of 60-80 Ah are most often found. That is, at 60 Ah, the device can produce a current of 1 Ampere for 60 hours. But this is in theory.
In practice, everything is different. As soon as the engine starts, the charge drops significantly. But it is compensated by the operation of the generator. Not all drivers drive a lot and often, and therefore the generator simply does not have time to replenish the entire charge. It has been proven that in most cases, cars are operated with constant undercharging.
Capacity can decrease under the influence of various factors:
- poor fastening, mechanical damage;
- electrical problems;
- violation of the integrity of electrical wiring;
- sulfation processes;
- driving around the city on short trips;
- low ambient temperature, etc.
Since most drivers drive in such conditions, it is imperative to periodically check the condition and charge of the battery.
Why is the battery draining?
Each battery has a certain capacity, which is measured in ampere-hours. That is, if the battery says that its capacity is 65 Ah, this means that the battery is capable of delivering a current of 1 ampere for 65 hours. However, in reality this is far from the case. The battery is discharged when starting the engine and operating electrical appliances, and is charged from the car generator.
This cycle is never complete, so over time the battery “gets used to” not using all the electricity accumulated in it, but only part of it.
Other factors may also influence the reduction in capacity. Among them:
- Mechanical damage. A poorly secured battery can crack, causing some of the electrolyte to leak out. Due to a strong discharge, the plates inside the battery short out and it stops working.
- Electrical fault. Too much current generated by the generator will lead to constant “boiling” of the battery. Too low will not have time to charge it completely.
- Sulfation. Inside the battery there are lead plates, which, under the influence of the acid contained in the electrolyte, begin to become covered with a white coating - lead sulfate. If this process is started, the battery will completely stop working.
- City use without additional charging. Frequent engine starts and a large number of switched on electricity consumers when driving in the urban cycle do not allow the battery to be fully charged. If you do not recharge it additionally from time to time, it will quickly fail.
- Reduced temperature. Every degree lower than +20 Celsius is minus 1% of the battery capacity. If it is -30 outside, this means that the battery can only operate at “half” of its rated power. If the old battery is partially discharged, even less so.
For these reasons, it is necessary to periodically inspect the battery. It is important to check both the condition of the battery itself and the conductive terminals. They need to be regularly cleaned of oxides and lubricated with a special lubricant.
Concept of CTC
Now we should understand in more detail the control and training cycle for batteries, since not everyone understands what it is and why it is carried out.
The batteries used in cars are lead-acid. They differ from each other in design features, additives used, and consistency of the electrolyte used. Therefore, there are AGM batteries, gel batteries, calcium batteries, etc.
The battery life is usually indicated by the manufacturer on the device body. However, you can often see figures within 5-10 years. This period seems quite acceptable, since the prospect of changing batteries every 7-8 years is encouraging. But the stated deadlines rarely coincide with the real ones. This is due to difficult working conditions, driving with constant undercharging. This affects cars used in the city and traveling short distances. Add to this low temperatures and neglect.
To minimize the cost of purchasing a new battery, you should do everything possible to extend the life of your existing battery. For this purpose, such a procedure as the CTC is provided.
A control-training cycle is a procedure that is carried out to restore discharged and old batteries. Its meaning is to completely discharge and then charge the device.
CTC allows you to partially restore characteristics and improve battery performance. You should not count on the same 100% efficiency as it was when you purchased it. But the battery will definitely serve you for an additional 2-3 years.
The recommended frequency of CTC is 2 times a year.
How to conduct battery training?
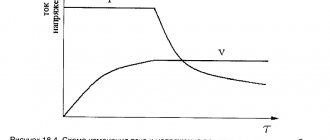
All training work with the battery is not carried out in the car. To do this, the battery is removed, charged using the charging technology with any available charger, after which the electrolyte density is measured in all sections of the battery. The average electrolyte density should be within 1.27 g/cm3. Checking the density is carried out with an aerometer, and some battery models have a built-in indicator that signals the level of electrolyte density. If the density is greater than the nominal value, add distilled water to the jar. If less, a ready-made electrolyte with a density of 1.4 g/cm3. Now the most important thing is to properly discharge the battery. To do this, it is connected to a powerful areostat, and the discharge parameters are controlled with an ammeter and a voltmeter. The connection diagram is so simple that we drew it ourselves. Here she is.
Now that the battery is connected, the ten-hour discharge process begins. The current value corresponds to 9% of the nominal battery capacity. That is, if the battery is 50Ah, then the discharge should be exactly 4.5A, if 60 - then 5.4A, a 75Ah battery is discharged with a current of 6.8A. It is very important to maintain the discharge current at a constant level until the voltage at the battery terminals drops to 10.3 V. Less than this value is not allowed.
Why do you need training?
Not everyone fully understands why such training is carried out on an old or dead car battery.
There are several main reasons:
- desire to postpone the purchase of a new expensive battery;
- increasing the service life of the used battery;
- resuscitation of a battery that was forgotten and found after a long time;
- restoring the characteristics of a battery that has been in use for a long time.
In some cases, when the battery has been lying in the garage for a couple of years or they simply forgot to remove it from the car, leaving it for long-term storage without the terminals removed, it is possible to restore the battery, which seems already condemned to disposal.
Correctly carried out training of an old car battery, when charging and discharging, allows the car owner to save money. Plus, the battery will somewhat restore its characteristics, and therefore the engine will start easier even in severe frosts.
Operational charging of a car battery
Periodic operational charging of a car battery is carried out, as a rule, with the battery removed from the car.
The battery removed from the car must first be carefully wiped, especially its upper part.
Before you put the battery on charge, you need to open all gas channels on the battery:
- remove the plugs
- and remove the lids of the jars
Then check the electrolyte level in the banks and, if necessary, bring it to normal.
And after that, connect the battery to a DC source.
It should be borne in mind that when the battery is partially discharged, the initial charge current when the rectifier is turned on can jump sharply upward. Therefore, the charging current should be adjusted to a value no higher than 1/10 of the battery capacity or less.
As the battery charges, the voltage will increase and the charging current will decrease.
The operational charge of the battery is carried out in the same way as the first charge.
The battery must be charged until abundant gas evolution occurs in all battery banks. After the start of abundant gas evolution, the voltage and density of the electrolyte should remain constant for 2 hours, this serves as a sign of the end of the charge.
Remember - you cannot charge with high current for more than 25 hours.
Because:
- The electrolyte will become very hot and boil away.
- The plates can lead from heating and they are shorted to each other.
Usually the normal time for a full charge is about 15 hours.
Sometimes during the operation of a car battery it is necessary to equalize the density of the electrolyte in the banks. This must be done with a small current. For example, the density of the electrolyte in different banks is different (1.23, 1.25). Then, by connecting the battery to the rectifier and turning it on, set the charging current to about 2A. (You need to use a voltmeter as a guide: the voltage should not be higher than 14V). This charging time is up to two days.
The same recharge of a car battery must be done after the battery has been discharged “to zero” by attempts to start the engine. Charging from a car generator will not bring the battery parameters to normal and such a charge should be done from a separate rectifier.
Such a charge must be made at the first opportunity and as early as possible, before sulfation of the plates into the battery begins.
Sequence of the CTC procedure
Many people carry out CTC of old batteries at home and successfully complete the tasks.
To perform this procedure, you will need to prepare:
- Charger;
- hydrometer;
- load of the required size;
- multimeter
Self-charging your own car battery using the CTC method quite often gives a positive result. But for this it is important to strictly follow the instructions and adhere to the given sequence.
To complete the training cycle, that is, charge and discharge worn-out batteries, you should learn how to use a multimeter.
The procedure itself includes 3 stages:
- pre-charge;
- control digit;
- charge.
It is important to perform each stage correctly. If during normal maintenance you only need to discharge the battery, then with CTC of the battery you need to know to what voltage to do this and when to start the reverse action.
Preliminary calculations are carried out for a specific battery to determine the exact load.
Preliminary stage
If you delve into the essence and study all the details, then the scheme for carrying out the CTC of a car battery will not seem so complicated. Therefore, many successfully do it with their own hands.
If you have a good quality factory charger, no problems will arise. Just connect the battery to the charger and wait for the process to complete.
The voltage up to which CTC will need to be carried out depends on the specific battery and the conditions of the cycle. Therefore, study its technical characteristics.
The charge is carried out according to the density of the electrolyte located inside and according to the voltage. When precharging the battery, focus on the following values:
- A voltage of 12.72 V corresponds to a density of 1.28 and indicates a 100% charge.
- A voltage of 12.5 V says the density is 1.24 and the charge is 75%.
- At 12.35 V the density will be 1.2. In this case, the battery is charged 50%.
- If 12.1 V, then the density is low, only 1.16, and the charge is only 25%.
These parameters, as well as the use of special formulas, will be relevant when using a simplified version of the charger. It is important to calculate the exact time.
How to properly discharge and charge a battery
To perform the control-training cycle correctly, it is important to follow the instructions for performing the operation.
The training cycle consists of several stages:
- Pre-charge the battery;
- Control digit;
- Final charge.
Each stage must be performed with all possible accuracy and quality. Before you start, be sure to complete all the necessary calculations based on the characteristics of your specific battery. To carry out a control discharge, a load of a certain, specific magnitude will be required.
Pre-charge battery
If you use a commercial battery charger, the procedure is quite simple. You just need to connect the battery to charging and wait for the process to complete. But even in this case, after charging, it is necessary to measure the battery with a hydrometer to ensure that the battery is fully charged, and, if necessary, equalize the level and density in all banks.
If charging is simplified, then you will have to apply calculations using the formula, although in this case there will not be any particular difficulty in charging the battery. A hydrometer measures the initial density of the electrolyte, taking into account the given battery capacity, and calculates the loss of capacity. You can find out what percentage the battery is charged by using the table.
Battery charge table by electrolyte density and voltage
| Voltage, V | Electrolyte density | Charge,% |
| 12,72 | 1,28 | 100 |
| 12,5 | 1,24 | 75 |
| 12,35 | 1,2 | 50 |
| 12,1 | 1,16 | 25 |
For example, the density is 1.16 g/cm3, which means that the battery is one quarter charged. Considering the battery capacity (for example, 60Ah), you can calculate the loss of capacity using the following formula:
Battery capacity multiplied by the charge (in%) and together divided by 100%
60Ah * 75% / 100% = 45 Ah
The charging voltage should be 1/10 of the battery capacity. To calculate the time required to fully charge the battery, you need to use the formula:
2*capacity loss/charging current=charging time
Charging current at 60Ah=6A.
The calculation using the formula taking into account all parameters is as follows:
t = 2 * 45Ah / 6A = 15h(hours)
It should be remembered that the calculated time may differ slightly from the actual time, so additionally check the voltage and density, indicators of 1.27-1.28 g/cm3 and a voltage of 12.7 volts indicate the end of the charge.
Correctly discharging the battery
To restore the functioning of the battery, it is necessary, paradoxically, to completely discharge the battery. However, the discharge must be controlled and produced at a strictly defined current strength.
It is necessary to create an electrical circuit from a consumer of electric current (a strictly defined capacity) as well as a voltmeter and an ammeter. It is necessary to discharge the battery with a current of the so-called 10-hour mode, the value of which is 9% - 10% of the battery capacity.
It is important to note that the battery discharge depends on the type of battery. The current value at CTC is also selected based on the type of battery.
To correctly select this value, you can refer to the table:
| Battery Type | Discharge current, Ampere | Battery Type | Discharge current, Ampere |
| 6ST - 140R | 12.6 | 6ST - 190 | 17.0 |
| 6ST - 45 | 4.2 | 6STEN – 140M | 12.6 |
| 6ST - 50 | 4.5 | 3MT - 12 | 1.2 |
| 6ST - 55 | 5.0 | 3MT - 8 | 0.7 |
| 6ST - 60 | 5.4 | 6MTS - 9 | 0.8 |
| 6ST - 75 | 6.8 | 6MTS - 22 | 2.0 |
| 6ST - 82 | 7.5 | 12ST - 85R | 8.0 |
| 6ST - 90 | 8.1 | 12ST - 70M | 7.0 |
| 6ST - 105 | 9.5 | 12ST - 70 | 7.0 |
| 6ST - 132 | 12.0 | 3ST - 150 | 13.5 |
| 6ST - 182 | 16.5 | 3ST - 215 | 19.5 |
The first number in the marking means the number of battery cells, ST means starter, and the numbers indicate the nominal capacity of the battery. We are interested in standard car batteries starting with: 6ST-...
In the case of a 60 battery, this is 5.4 amperes. You can simply purchase a regular car light bulb that is as close in power as possible.
- You can select it by calculating the required characteristics of the lamp using the formula: P = I * U
where: P is the power measured in W, U is the voltage (12 Volts), and I is the current required for us.
- Substituting all the values, we find: 5.4 A * 12v = 64.8 W.
In other words, to carry out CTC, a 60 Ah battery will require a 65 watt light bulb.
When discharging, be sure to monitor the condition of the battery!
- The electrolyte temperature at the beginning of the operation should be between 18 and 27 °C. When the battery is discharged, you need to carefully monitor the constant current.
- Voltage and temperature are measured before the battery is set to discharge, then measured every 2 hours.
- When the voltage drops to 1.85 V, measurements are taken every 15 minutes.
- As soon as the voltage drops to 1.75 V, the discharge process must be monitored continuously.
- At a voltage of 1.7 V, the discharge should be completed.
If the battery discharge time has decreased significantly, this means that the battery parameters have noticeably deteriorated. The battery will work, but in accordance with the remaining capacity.
To calculate the residual capacity of the battery, you can use the formula:
Q=current value * discharge (in hours)
For example, the nameplate capacity is 60 Ah, and it took 6 hours to discharge it with a current of 5.4 amperes. The amount of residual capacity can be calculated simply by multiplying using the formula:
Q = 5.4 * 6 = 32.4 Ah.
The resulting actual capacity is much less than that indicated in the battery's passport, which indicates that such a battery gradually fails.
Important! When performing a control discharge, be sure to note the start and end times and record the initial and final temperatures.
Why does the charge drop?
All batteries have a certain capacity, stated in Ah. In passenger cars, batteries of 60-80 Ah are most often found. That is, at 60 Ah, the device can produce a current of 1 Ampere for 60 hours. But this is in theory.
In practice, everything is different. As soon as the engine starts, the charge drops significantly. But it is compensated by the operation of the generator. Not all drivers drive a lot and often, and therefore the generator simply does not have time to replenish the entire charge. It has been proven that in most cases, cars are operated with constant undercharging.
Capacity can decrease under the influence of various factors:
- poor fastening, mechanical damage;
- electrical problems;
- violation of the integrity of electrical wiring;
- sulfation processes;
- driving around the city on short trips;
- low ambient temperature, etc.
Since most drivers drive in such conditions, it is imperative to periodically check the condition and charge of the battery.
Design and principle of operation of the battery
The design of the battery is quite reliable, and yet there are cases of damage to batteries either due to careless use or improper maintenance.
Further use of the battery in a car if it is damaged is impossible. But if a relatively new battery has been damaged, you should not replace it immediately; you can try to restore it.
First, let's figure out what the design of an acid battery is.
If you look at it externally, it consists of a closed plastic case with two terminals.
Structurally, the batteries are serviceable or maintenance-free.
Serviceable batteries have plugged holes in the upper part of the battery case.
Maintenance-free batteries are not equipped with such holes; there is only a small hole for gases to escape.
Inside, the body is divided into 6 sections called banks. These banks house the working elements of the battery - a set of positive and negative lead plates with active mass applied to them.
They are located variably, that is, there is a positive plate, next to it there is a negative one, then there is a positive one again.
Additionally, to prevent possible contact of these plates, a separator is located between them.
The plates are combined into blocks, each block has an output jumper - a bracket, which is connected to the bridge. Using a barrette, the blocks of each can are connected to each other into a common bridge with output to a terminal.
The battery releases electricity through chemical reactions, so the jars are filled with electrolyte - a strictly dosed solution of acid with distilled water.
The battery itself does not generate electricity; it is, in fact, just a storage unit for electricity.
When charging the battery, the electrical energy supplied to the terminals from the charger or generator is converted into chemical energy. And when discharged, the opposite effect is produced.
It all works like this: when an energy consumer is connected to a battery, the spongy lead of the negative plates reacts with the lead dioxide of the positive plate and the electrolyte.
There is chemistry between them. reaction, resulting in the release of electricity, which is consumed by the consumer. In this case, a layer of lead sulfate appears on the negative plates.
When charging a battery, the reverse process occurs, as a result of which the layer of sulfate that appears dissolves in the electrolyte, and a layer of lead dioxide forms on the positive plates.
To put it simply, when the battery is discharged from the positive plates, lead particles are due to chemicals. reactions are transferred to negative ones. And when the battery is charged, these particles return back to the positive plates.
All this is accompanied by the release or consumption of electrical energy.
Determining the cause of the discharge
We need to determine where the charge goes: whether it is being “eaten off” by background processes and services, or whether the discharge is caused by a decrease in battery capacity due to wear and tear. This is quite easy to do through the built-in battery usage statistics function. Starting with iOS 7.0, we have not only meager usage and expectation figures (although there are plenty of them), but even detailed statistics on applications.
The bottom line is that the iPhone and iPad should not be discharged in standby mode, which means the standby time from the statistics menu should be significantly longer than the use time (even though the device is at rest).
News Media2Battery discharge and charging modes
During long-term (several months) storage of car batteries, they self-discharge, and therefore it is recommended to recharge the batteries at least once a month. However, conventional recharging is not able to prevent sulfation of the plates, which leads to a decrease in battery capacity and a decrease in its service life. In order to eliminate these undesirable phenomena, it is recommended to train the battery from time to time:
discharging it with a current, in amperes, numerically equal to 1/20 of the nominal capacity, expressed in ampere-hours, to a voltage of 10.5 V, and subsequent charging to a voltage of 14.2 ... 14.5 V. This charge-discharge cycle can be repeated repeatedly, if the battery is heavily sulfated or has been in a half-discharged state for a long time.
A charger/discharge device is required for this purpose.
— discharge the battery to a voltage of 10.5 V;
— automatically start charging after discharging ends;
— charge with an asymmetric current with a ratio of charge and discharge components equal to 10;
- stop charging the battery when the voltage at the battery terminals reaches the value
14.2…14.5 V, which corresponds to informing the battery of its full nominal capacity;
— voltage control occurs at a time when charging current does not flow through the battery;
— stop discharging the battery when the mains voltage is lost;
1. When charging the battery with a constant current that does not change during the charging process, it is stopped manually after a certain time. Many of the cheapest chargers are oriented towards this mode. The charging current in them is usually I = 0.1 E, where I is the charging current in amperes, and E is the battery capacity in ampere hours. In this mode, the capacitive efficiency of the battery is taken to be 2/3 and, accordingly, the charging duration is set to 15 hours. The low current charging mode (it can be less than 0.1 E with a corresponding increase in charging duration) is remarkable in that even with significant overcharging the battery will not be damaged, or at least will not explode :).
2. The battery is charged with a direct current many times greater than 0.1 E (10...20 times). Charging stops automatically after a specified (shorter) time has elapsed. In such intensive charging mode, the following must be observed. Firstly, the battery must first be discharged (usually to 1V per cell); secondly, a strict dependence of the charging duration on the set value of the charging current must be ensured and, thirdly, its emergency shutdown must be ensured (for example, due to overheating of the case). In theory, many chargers that have appeared on our market fall into this category, but, unfortunately, not all of them provide adequate safety.
3. The charging current is not necessarily constant. Charging of the battery stops when its temperature increases. This method has serious disadvantages (the battery is almost always overcharged, the thermal contact is unreliable, etc.) and is used, as a rule, only for emergency shutdown of the battery.
4. Charging current - fixed, many times, usually exceeding 0.1 E. When the battery reaches the specified voltage, charging ends automatically. This principle has long been used in the best chargers, displacing the low-current battery charging system. Setting the threshold voltage is very critical here. Usually its value is chosen within the range of 1.45...1.55V per battery bank, more often - 1.48V. The threshold voltage also depends on the ambient temperature and the “age” of the battery. A constant charging current is, generally speaking, not required here. But this simplifies the accounting of losses on the supply wires. If, due to their failure to take them into account, a low threshold voltage is set on the battery, this will result in a lack of charge, and if it is set only one millivolt higher than the real one, the process of charging the battery will never end. Or rather, it will end with the battery either overheating - with a low charging current, or exploding - with a high one. To avoid this, some chargers, upon reaching a voltage slightly less than the threshold, switch to recharging the battery with a safe current, which completes it.
5. The charging process is controlled by the rate at which the voltage on the battery increases: it increases quickly just before it is completed. Having tracked this moment, the charger reduces the high charging current (it reaches up to 2 E) to a small, safe one, with which charging is completed. For the reasons stated in paragraph 4, it is also better to have both of these currents fixed and not changing over time. This method began to attract attention with the advent of the specialized U2402B microcircuit.
6. As in the previous case, when charging with direct current, the condition of the battery is determined by the voltage surge. To obtain good characteristics, charging is carried out with a current of at least 2 E.
7. Now let’s consider the case of deep discharge: in this case, the battery must be charged with a reduced voltage (12V..13V). The acid from the electrolyte has gone into the plates; if you give the rated charging current, the process of electrolysis of water will begin. It is necessary to ensure that the current at the beginning of the charge does not exceed 1/20 of its capacity in Ampere hours (in principle, this should happen automatically, in contrast to the situation when 14.4 V is immediately supplied to the terminals). If there is more, reduce the tension. The current will gradually increase - this is normal. This acid comes out from the depths of the plates, lead sulfate gives an influx of acid, and the density of the electrolyte increases. When the current rises to 1/10 of the battery capacity, or even more, and it’s very good - when after this rise it even begins to decrease - then you can switch to the charging process described above, i.e. set the voltage to 14.4V.
8. Chargers
The most interesting today is the ULTRA DUO charger, in which charging ends when the voltage surges on the battery.
In the MULTI-CHARGE-A-MATIC CG-325 charger from HITEC, the end of charging is determined as in the previous case, but charging is carried out with a set constant current (maximum 4.5A). In addition to such usual functions as discharging the battery before charging, checking its capacity, reverse polarity protection, monitoring the charging duration and sound signaling of its end, this device, thanks to the built-in voltage converter, can charge ten series-connected nickel-cadmium batteries from a 12-volt car battery ( the voltage on which in the charged state reaches 16V). This will be appreciated, first of all, by motorists who use portable radios.
According to established terminology, battery charging can be very fast (up to 15 minutes), fast (up to 1 hour), accelerated (up to 3...4 hours), normal (12 to 16 hours) and slow. The actual battery capacity depends on temperature and charging and discharging current values. The highest measured capacity is obtained when the battery is charged with a high current and discharged with a low current.
Now it became clear to me that the car designers were right a thousand times over, using ordinary electric batteries on them, and not capacitors or superconducting magnets.
Indeed, car batteries can store energy for months, and in fairly large quantities.
9. From history
The history of electric batteries dates back to the famous experiment carried out by Italian physicist Alessandro Volta in 1799. The scientist dipped copper and zinc electrodes into dilute sulfuric acid and discovered that a potential difference arose between the electrodes. By connecting the electrodes with a conductor - a wire, Volta received an electric current in it. Thus, he proved that various metals placed in acid solutions form a source of current.
This was the world's first galvanic cell, later named after the Italian physicist and physician Luigi Galvani, who, even before Volta, noticed the appearance of current when two different metals interacted in a conducting liquid - an electrolyte.
True, there is information that galvanic cells existed in ancient times. During archaeological excavations, clay jugs with electrode-like cylinders made of different metals were found, and some scientists believe that wine or vinegar served as the electrolyte. And it’s as if, with the help of these elements, the ancient craftsmen knew how to make electroplating coatings: for example, they applied the thinnest film of gold to jewelry.
One way or another, Volta’s great merit is that he not only built a galvanic cell, but also explained its action, which, for obvious reasons, the ancients could not do.
The Volta element produced very little voltage. To increase it, they began to make batteries from copper and zinc plates, lined with gaskets soaked in sulfuric acid. These batteries, called voltaic columns, already provided quite a high voltage. After Volta, many scientists - Leclanche, Grenet, Daniel, Grove and others - developed their own, more and more advanced galvanic elements. The Leclanche cell, for example, served as the prototype for modern dry-cell batteries used to power flashlights, radios, electrified toys and other devices. The electrodes of such batteries, as Leclanche once used, are solid - a zinc cup and a graphite rod. But the electrolyte is no longer liquid. After all, liquid can spill at any time, and making the element airtight is expensive and difficult. So they replaced the liquid with a jelly-like electrolyte. The result is a convenient and practical source of electricity.
Lead-acid batteries are very economical, but they are also capricious, often deteriorate, and are short-lived. In addition, lead is a relatively rare and expensive metal, and acid is dangerous to handle. Naturally, scientists began to look for new materials and new principles of battery operation. This is how the second main type of electrochemical batteries arose - alkaline batteries. Their creation is closely connected with the name of the famous American scientist and inventor Thomas Edison.
In these batteries, the electrolyte is no longer acid, but alkali - a 20% solution of caustic potassium. The plates are made of steel grids with pockets in them. The positive plates have pockets filled with a mixture containing nickel oxide, while the negative plates have pockets filled with cadmium sponge. The alkaline battery has a steel body, which makes the device more durable.
Alkaline batteries are more expensive than acid batteries and less economical. But, despite this, their positive qualities prevail - they are unpretentious, strong, durable. Therefore, they are increasingly becoming involved in technology. For example, trolleybuses use just such storage devices. They can be seen in transistor radios, telephones, hearing aids, flashlights, and other devices. Many radio devices contain miniature batteries, also alkaline, called “button” batteries, since they look like a button. Their value lies in the fact that they are hermetically sealed, completely insensitive to overcharging and overdischarge, and do not require maintenance. Ordinary large batteries cannot boast of this.
Some communications satellites and space stations use very expensive, but excellent in their characteristics, silver-zinc alkaline batteries. They don’t care about high currents or low, down to minus 60 degrees, temperatures. The energy density accumulated in them is five times higher than that of acid batteries, and the power density is twice as high.
Silver-zinc batteries are good for everyone, even now put them in your car. The weight of the battery to cover a hundred-kilometer path will not exceed one hundred kilograms...
For a battery to become truly widespread and promising, it must contain materials that are abundant on Earth.
Now scientists pin their hopes on an unusual at first glance battery, which uses galvanic pairs “sulfur-sodium” and “chlorine-lithium”. Metals - sodium or lithium - are molten there, their temperature reaches several hundred degrees. Molten sodium combines with hot liquid sulfur in the battery, and lithium interacts with hot gas - chlorine. Due to the fact that the contents of such batteries are heated to 300...800 degrees during operation, they are called hot.
For some reason, what was happening inside the hot batteries immediately reminded me of the mythological hell about which I read a lot as a child. It was enough to imagine molten sulfur in which molten sodium is “cooked”, the same sodium that ignites and even explodes from water! There is nothing to say about chlorine - it is one of the most poisonous gases, extremely active even at room temperature, but what will happen at eight hundred degrees! It’s no wonder that scientists have been struggling for a year to create a housing for this “hellish” drive - few materials can withstand such filling.
However, to the credit of hot batteries, at their low cost, they develop an energy density ten times greater than lead-acid batteries, and their power density is much higher. If lead-acid batteries accumulate 64 kilojoules of energy per kilogram of their mass, and alkaline batteries - 110, then hot sodium sulfur batteries - 400...700 kilojoules!
For a car to run 100 kilometers, only 50 kilograms of a sulfur-sodium battery would be enough. 150 kilograms per 300 kilometers are good results. But... hot batteries must be warmed up before starting work; their shell cannot withstand the “hellish” contents for long. And even if a car with this battery crashes, you wouldn’t want anyone to be present, even as a spectator.
The new copper-lithium batteries have a calmer “character”. They have a copper alloy cathode and a porous lithium anode. The electrolyte is organic, with high electrical conductivity. The energy density in prototypes of these batteries is one and a half times higher than that of silver-zinc batteries, but, most importantly, they can achieve high power densities. If, instead of copper, you take a nickel fluoride compound, then the process of charging the battery can be greatly reduced, to just a few minutes, which is also very significant.
Batteries based on zinc and... ordinary air are interesting. The zinc anode here is simply oxidized by atmospheric oxygen, so the entire energy reserve in the battery is determined only by the amount of zinc. The cathode is made of porous nickel and is almost not consumed, and the anode, as it wears out, is replaced with a new one or restored by passing a charging current.
The peculiarity of these batteries is that they can operate both in the mode of batteries and in the mode of conventional galvanic cells, simply “burning” zinc in the oxygen of the air. It is in this case that the zinc anodes have to be replaced, but the energy density of the element is almost twice that of a battery.
However, no matter how good the record-breaking batteries described above are, experts still believe that the problem of creating a modern electric car with a range of 120...150 kilometers should be solved not by them, but by cheap and abundant nickel-zinc batteries. In terms of energy density and power, such batteries are between conventional and silver-zinc batteries. They arose as a result of replacing expensive silver in silver-zinc elements with relatively cheap nickel. New, roll, battery types
Roll cell production technology allows Optima batteries to combine the advantages of starter and traction batteries. Optima batteries withstand multiple discharge and charge cycles without compromising capacity and are ideal for seasonal use, as they have a low degree of self-discharge. All battery models do not require maintenance and have a durable sealed housing. They can work in any position without leaking electrolyte.
Optima batteries are distinguished by color coding on the top of the battery. Minimal. The capacity of batteries with a red top RT (56 A/h, 830 ampere, 12 V, 17.7 kg, 245x172x199 mm 12 thousand starting cycles) is used as a starter, convenient for frequent engine starts. Batteries with a yellow top YT (60 A/h, 750 A, 12V, 19.5 kg, 245x172x199 mm) are used on vehicles equipped with additional powerful consumers of electricity - winches, amplifiers, engine heaters. Maintains a sufficiently high voltage at long-term discharge currents to a much greater extent than the current of a conventional battery. Batteries BT (Blye Top) combine the qualities of starter and traction batteries. Blue battery capacity 75 Ah, dimensions 254x172x199
The battery is assembled from roll cells. The element is a roll; between layers of chemically pure lead, rolled into a roll, a microporous fiber impregnated with an electrolyte is laid. The characteristics of Optima batteries retain their characteristics in the range from -60 to +80 degrees C. The durability of the batteries is 4 times longer than that of conventional batteries.
The capacity of the Optima battery during the initial period of operation is approximately 85% of the nominal value. During operation, after 17-19 discharge/charge cycles, the capacity reaches its nominal value. To prepare the battery for operation, it is proposed to cycle (train) the battery in accordance with the following recommendations:
1. Discharge a fully charged battery to a voltage of 10.5V
2. Charge the battery for 16 hours with a current of 4A.
3. Repeat steps 1 and 2 three times.
There are several charging modes for Optima traction batteries. These are batteries with yellow and blue tops. Below are the charging methods.
1.In a car at a constant voltage from the generator 14.2 - 15.0 V.
2. From a charger at constant voltage. Voltage 14.2-15.0 V, current 10A. Duration of charge until the current drops below 0.2A
3. When using the battery as a traction battery, it should be charged in three stages:
— 1st stage: charge with a current of 25A until the voltage reaches 14.7V
— Stage 2: continue charging at a fixed voltage of 14.7 until the charge current drops to less than 1A.
— Stage 3: Raise the charge current to 2A and charge for 1 hour. Voltage doesn't matter.
4. There is an accelerated charging technique. Charge with a voltage of 15.6 V without limiting the charging current. Monitor the temperature of the battery case. The temperature should not exceed 50 degrees. The duration is determined empirically to ensure charging of 110-120% of the used capacity (please note - not the nominal one).
5. Stationary use (floating charging).
When working in buffer power or during storage. Charge voltage from 13.2 to 13.6V with a current of 120 milliamps. Related materials:
Source:
Concept of CTC
Now we should understand in more detail the control and training cycle for batteries, since not everyone understands what it is and why it is carried out.
The batteries used in cars are lead-acid. They differ from each other in design features, additives used, and consistency of the electrolyte used. Therefore, there are AGM batteries, gel batteries, calcium batteries, etc.
The battery life is usually indicated by the manufacturer on the device body. However, you can often see figures within 5-10 years. This period seems quite acceptable, since the prospect of changing batteries every 7-8 years is encouraging. But the stated deadlines rarely coincide with the real ones. This is due to difficult working conditions, driving with constant undercharging. This affects cars used in the city and traveling short distances. Add to this low temperatures and neglect.
To minimize the cost of purchasing a new battery, you should do everything possible to extend the life of your existing battery. For this purpose, such a procedure as the CTC is provided.
A control-training cycle is a procedure that is carried out to restore discharged and old batteries. Its meaning is to completely discharge and then charge the device.
CTC allows you to partially restore characteristics and improve battery performance. You should not count on the same 100% efficiency as it was when you purchased it. But the battery will definitely serve you for an additional 2-3 years.
The recommended frequency of CTC is 2 times a year.
Continue reading
- Comparison of batteries Comparison of batteries from different manufacturers When designing an autonomous or backup power supply system, the question is always: which batteries are best to choose? There are many brands, types, and models of rechargeable batteries on the market, and it can be very difficult to understand them. Our clients often ask the question...
- Prosolar-R batteries Prosolar-R batteries Prosolar-R batteries of the D (AGM) and DG (gel) series are specially designed for operation of autonomous and backup power supply systems. Over the several years that we have been using these batteries in power supply systems, they have proven to be much better than general purpose batteries. This…
- Rechargeable batteries. Educational program How to extend the life of lead-acid batteries? It is often difficult to directly use the energy generated by solar, wind or microhydroelectric installations. Therefore, electricity is usually stored in special batteries for later use. These batteries very often work on the same principle as...
- Storage technologies Energy storage technologies in autonomous power supply systems Based on materials from the site: modernoutpost.com This note contains general tips on choosing batteries for systems with renewable energy sources. The article covers 3 main technologies: lithium-ion, nickel-metal hydride and lead-acid (AGM, or Gel). We will try…
- Selecting the Cable Size Selecting the Cable Size The cables connecting the inverter and batteries carry a very high current. Therefore, it is necessary to correctly select the cable cross-section based on the maximum currents that the inverter can consume. It is very important that the connections are reliable and have low resistance. For that,…
- Types of lead-acid batteries Types of lead-acid batteries Serial production and mass operation of lead-acid batteries began at the end of the 19th century. At the beginning of the 20th century, they began to be widely used in cars, further developing the scope of their application, easily crossed the millennium mark and are still...
At first glance, this is an absurd question. But only at first glance. If the battery is no longer as strong as it was immediately after purchase, it can be given a “second wind” by sequentially discharging and then fully charging. This procedure is even desirable; it should be carried out before the cold season.
Let’s say right away: “discharging a battery” does not mean subjecting it to a deep discharge.
That is, turning on all on-board systems with the engine off and waiting for the battery to give up all its energy is a dead end. A calcium battery will not survive such a procedure at all; batteries of other classes will not react very well to it either. It is necessary to discharge to clearly defined electrical parameters. Namely: 10.3 V on the battery as a whole and 1.7 V on each bank. We repeat: the procedure is only suitable for low-antimony batteries; it is better to limit other classes of batteries to a normal charge. But after such a reboot, the batteries of the lead-antimony system can work as in their younger years.
To begin with, how to discharge?
There are several options, but all of them require a multimeter, voltmeter or charger with voltage fixation at the terminals. We connect the equipment to the connected battery and “turn on” the high and low beams. There is no need to connect all the electronics on board - the battery does not like both fast charging and fast discharge. So, we are waiting for the coveted numbers. Afterwards, remove the battery and charge it. If you are near the car without your hand, remove the battery and perform all the manipulations at home. The best discharge option is to connect a 12 V, 60 W light bulb to the battery. These are installed in car headlights. We’ll just get the required 5 A of consumption. At the same time, you can calculate the real capacity of your battery. If you don’t have a light bulb at hand, any 5-7 A consumer will do.
It’s even more convenient if there is a discharge function on the charger. This option is common on more or less variable devices. All that remains is to set the lower voltage limit, after reaching which the charger will stop discharging.
Next is charging.
It needs to start immediately. If it is not possible to immediately charge the battery, do not even start the discharge procedure - you will destroy the battery. In a completely discharged battery, literally after a couple of hours the sulfation process begins - coating the plates with lead sulfate. The deeper the sulfation, the less likely the battery will work at all. So, immediately after the discharge - charging. Preferably with low currents. If you are not going anywhere in the next few days, that’s ideal. Set the charger to a current of 1.5 to 5 A and wait until the container is completely filled. At 1.5 A, charging may take 2-3 days (depending on the battery capacity), but charging with low currents partially desulfates the battery, and therefore restores its capacity.
Up to two such CTC cycles can be performed at a time. But even one may be enough for the battery to recover and serve you faithfully for another couple of years.
The average service life of a modern lead-acid battery for a car is from 5 to 7 years. This is only the case if the owner complies with all the manufacturer’s requirements for maintenance and operation of the battery. You can't always predict everything, and death comes suddenly. This also applies to the battery. In order not to throw away extra money buying a new battery, it is enough to pay attention to it literally twice a year. Then it will serve for a reasonable 7 years, and you can even remain in reserve. For this you need nothing at all - training the car battery.
Why train your battery?
The battery needs to be regularly diagnosed: check its external condition, the voltage it produces, and the electrolyte level in it. Typically, such a check is combined with a seasonal technical inspection. If the battery does not charge well and runs out quickly, this is the first sign that it is time to conduct a control and training cycle. At home, battery CTC can be carried out in any room where there is an exhaust hood. The control and training cycle of acid batteries can be done in the garage, but always with the battery removed and disconnected from all consumers.
Carrying out CTC of batteries can also be entrusted to specialists. However, this procedure is not at all difficult, and it is quite possible to do it yourself. For this you will need:
- Charger;
- two wires;
- voltmeter;
- rheostat or light bulb of suitable power;
- a clock to keep track of time.
It must be remembered that when charging batteries, hydrogen is released, so the room in which work with the battery is carried out must be well ventilated.
You should refrain from smoking and using open fire. Battery electrolyte is a strong acid, and when working with it, all necessary precautions must be taken to protect the skin, eyes and respiratory system.
Procedure for carrying out the CTC
The control and training cycle of rechargeable batteries begins with charging. The battery should be fully charged using the charger. After this, you need to check the density of the electrolyte using a hydrometer. Its normal value is in the range of 1.27 - 1.28. If the density is higher than normal, add distilled water. The electrolyte level should be 10-15 mm above the level of the plates. If the density is below normal, it is better to replace the electrolyte with a new one.
The next stage of the battery CTC is its complete discharge. It is produced by the so-called ten-hour current. Its value is defined as 10% of the nominal battery capacity. If the battery capacity is indicated, for example, 55 A*h, then it must be discharged with a current of 55/10 = 5.5 amperes. In order to accurately adjust the current value, a rheostat must be included in the circuit. However, in the absence of it, you can use a car light bulb that is suitable in power. 5.5A*12V=66 W.
The light bulb is connected to the battery as a load, and a voltmeter is connected in parallel. The discharge level should be constantly monitored so as not to discharge the battery to zero. Some batteries may not be able to withstand too much discharge. The battery is discharged until the voltage at the terminals is 10.3 V. It cannot be discharged below.
Why do you need training?
Not everyone fully understands why such training is carried out on an old or dead car battery.
There are several main reasons:
- desire to postpone the purchase of a new expensive battery;
- increasing the service life of the used battery;
- resuscitation of a battery that was forgotten and found after a long time;
- restoring the characteristics of a battery that has been in use for a long time.
In some cases, when the battery has been lying in the garage for a couple of years or they simply forgot to remove it from the car, leaving it for long-term storage without the terminals removed, it is possible to restore the battery, which seems already condemned to disposal.
Correctly carried out training of an old car battery, when charging and discharging, allows the car owner to save money. Plus, the battery will somewhat restore its characteristics, and therefore the engine will start easier even in severe frosts.
Subtleties of training battery
The process is quite subtle, requiring attention, but extremely effective. We note the time from the beginning of the discharge. The first freeze occurs at the very beginning of the discharge cycle, and care must be taken to ensure that the electrolyte does not overheat. Subsequent measurements are carried out no earlier than 3-4 hours later. The main thing is not to miss the moment when the voltage drops to 11V. After this threshold, measurements are made every 10-15 minutes until the voltage value drops to 10.2-10.3V. The time spent discharging the battery with a given current will speak eloquently about the true capacity of the battery. The shorter the discharge time, the lower the actual capacity. This is easy to calculate. The discharge current is simply multiplied by the time spent discharging to 10.2 volts. For example, the battery has a capacity of 90 Ah, therefore, the discharge current should be exactly 8.1 A. According to measurements, the battery was discharged in 6 hours. It turns out 6x8.1 = 48.6. Consequently, after the first discharge cycle, the battery has a capacity of 48.6 Ah, which is almost half the nominal value. Now you need to repeat the procedure for charging the battery using the standard method or pulse current until fully charged, and then carry out a training-restorative discharge using the same scheme. A similar result can be achieved by restoring the battery with pulsed current, in manual mode, as described above, the result is guaranteed. After 3-4 such cycles, the actual capacity of the battery will increase beyond the nominal in 90% of cases, so do not rush to recycle old batteries. Sources used:
- https://kat.ru/mdk.01.01_elektro/11-cikly_zaryad_razryad/index.shtml
- https://istochnikipitaniy.ru/stati/kolichestvo-tsiklov-zaryada-u-akkumulyatorov.html
- https://www.solarhome.ru/basics/batteries/ab_params.htm
- https://akb-moscow.ru/kak-pravilno-razryadit-akkumulyator-avtomobilya/
- https://myfords.ru/kak-prodlit-zhizn-akkumulyatoru
Battery control and training cycle (CTC)
using UTAB 12-60/20 and UZPS 48-20
The importance of conducting a battery control-training cycle (CTC) is well known.
In general, the CTC of a battery consists of carrying out the “CHARGE-DISCHARGE-CHARGE”
and takes quite a long time.
To facilitate the CTC process (reduce labor costs), we offer a set of equipment for an automated battery testing complex, using the UZPS 48-20 charging and feeding device and the UTAB 12-60/20 battery testing device.
The listed devices have the ability to work together with each other; their joint operation is ensured using a signal cable.
Let's consider the operation of the complex using the example of a TPL 121500 battery (12V, 150Ah).
The composition of the complex and the connection of devices to the battery are shown in the figure. We don't need a hydrometer because... The battery is sealed.
KTC (full battery charge)
The modern market is oversaturated with automatic chargers. Our company offers battery chargers of its own production, which can be purchased at a competitive price. When using such devices, the CTC procedure is much easier. Charge the battery and wait until it is fully charged. To be sure that the battery is charged, check the electrolyte level using a hydrometer.
The battery charging time is calculated using this formula.
Signs of the end of the charge: constant electrolyte density and voltage for 2 hours (temperature, as well as electrolyte density and voltage at the end of the charge are measured after an hour).
CTC (battery discharge)
To discharge the battery, it is advisable to use discharge devices. During the process, the battery voltage is periodically checked - the first measurement at the beginning, the next one after four hours.
It is important to remember that you cannot leave a discharged battery for a long time!
After the battery is discharged, you need to charge it again in the same way as in the beginning.
On average, the CTC process can take two days. It is recommended to carry out the entire CTC cycle 2-3 times.
Our company produces a range of devices for servicing batteries of all types. All products produced by our enterprise are made using high technology, which contributes to uninterrupted operation for a long time.
Sequence of the CTC procedure
Many people carry out CTC of old batteries at home and successfully complete the tasks.
To perform this procedure, you will need to prepare:
- Charger;
- hydrometer;
- load of the required size;
- multimeter
Self-charging your own car battery using the CTC method quite often gives a positive result. But for this it is important to strictly follow the instructions and adhere to the given sequence.
To complete the training cycle, that is, charge and discharge worn-out batteries, you should learn how to use a multimeter.
The procedure itself includes 3 stages:
- pre-charge;
- control digit;
- charge.
It is important to perform each stage correctly. If during normal maintenance you only need to discharge the battery, then with CTC of the battery you need to know to what voltage to do this and when to start the reverse action.
Preliminary calculations are carried out for a specific battery to determine the exact load.
Preliminary stage
If you delve into the essence and study all the details, then the scheme for carrying out the CTC of a car battery will not seem so complicated. Therefore, many successfully do it with their own hands.
What is a control-training cycle?
If the battery is very tired from life or has not been used for a long time, only the CTC - control-training cycle - can help it. It will also help you get to know the battery that was in, say, a newly purchased car, and determine its approximate resource and lifespan. The whole operation is easily carried out with your own hands without complex equipment and encyclopedic knowledge. It is enough to finish reading this manual, and the battery can live a new life, breathe deeply and delight the owner with a stable 12 V. Experienced motorists recommend training and restoring the car battery at least once a year, with the exception of new batteries. The training cycle includes a full battery charge, a test discharge of the battery, and then recharging.
How does temperature affect the charging of a lead-acid battery?
Everything that is written above applies to charging a lead battery at a temperature of 20 degrees Celsius, and at other temperatures it is necessary to introduce temperature compensation for the charging voltage. Charging a lead battery is possible in the temperature range from -15 ° C to +40 ° C. As the temperature increases, the charging voltage should be lower than normal to avoid overcharging. And if the battery is charged at a low temperature, the charging voltage must be increased to avoid undercharging. Temperature compensation of -3 mV/°C is generally recommended.
Capacity restoration
Battery charge indicator
It will take several times in a row to restore the battery to ideal condition. By the time the first and all subsequent charges are completed, the battery power increases, and, accordingly, the charge perception increases. While the storage element is not charging, the potentials of the electrodes, which are located in different parts of the battery plates, are equalized, as a result of which the compacted electrolyte diffusions from the pores of the plates and the maximum voltage decreases. Therefore, during repeated charges the density increases.
What most often shortens the life of a car battery?
- Electrolyte evaporation. When operating a car, especially in the summer, when it is especially hot under the hood, water evaporates from the electrolyte. As a result, the electrolyte level drops and the battery plates are exposed. This very quickly leads to their sulfation;
- The battery is not always charged. This situation is observed when trips are short and there are a large number of current consumers in the car;
- Low electrolyte density. If the density of the electrolyte is low, it can simply freeze in cold weather. This threatens that the plates in the jars will be warped. In this case, the battery is immediately sent to a landfill;
- Faulty electrical wiring in the car. Problems in the vehicle's on-board network significantly reduce the life of the vehicle's battery;
- Inaccurate operation. Using a loose or poorly secured battery may result in impacts, overturning, etc. As a result, the case and terminals may be damaged, and a short circuit may occur.